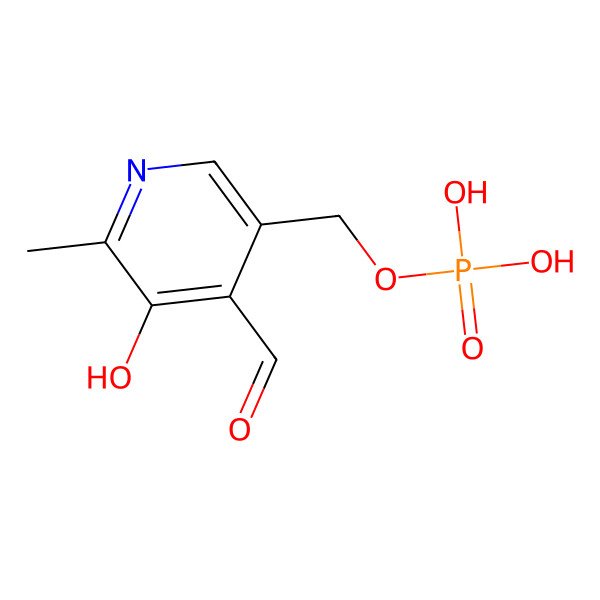
| 54-47-7 |
| Codecarboxylase |
| pyridoxal 5'-phosphate |
| pyridoxal 5-phosphate |
| Pyridoxyl phosphate |
| Pyridoxal P |
| Pyridoxal-5'-phosphate |
| Biosechs |
| Hairoxal |
| Pidopidon |
| Pyromijin |
| Vitazechs |
| (4-formyl-5-hydroxy-6-methylpyridin-3-yl)methyl dihydrogen phosphate |
| Hiadelon |
| Himitan |
| Phosphopyridoxal |
| 853645-22-4 |
| PYRIDOXAL-5-PHOSPHATE |
| Pal-P |
| Sechvitan |
| Pydoxal |
| Piodel |
| Apolon B6 |
| HI-Pyridoxin |
| pyridoxal-P |
| Phosphopyridoxal coenzyme |
| Vitahexin P |
| Hexermin P |
| Coenzyme B6 |
| Pyridoxal monophosphate |
| Pyridoxaldehyde phosphate |
| Apolon B(sub 6) |
| PLP |
| Phosphoridoxal coenzyme |
| Pyridoxal 5'-phosphate hydrate |
| Vitamin B6 phosphate |
| pyridoxal-5P |
| Pyridoxal 5'-(dihydrogen phosphate) |
| Pyridoxal 5-monophosphoric acid ester |
| Vitahexin-P |
| Hexermin-P |
| Pyridoxal phosphate [JAN] |
| 3-Hydroxy-2-methyl-5-((phosphonooxy)methyl)-4-pyridinecarboxaldehyde |
| 4-Pyridinecarboxaldehyde, 3-hydroxy-2-methyl-5-((phosphonooxy)methyl)- |
| Sechvitan, Vitahexin P |
| Pyridoxal 5/'-phosphate (hydrate) |
| Pyridoxal-5-monophosphate |
| EINECS 200-208-3 |
| Pyridoxal, 5-(dihydrogen phosphate) |
| NSC 82388 |
| Pyridoxal phosphate (6CI) |
| 3-Hydroxy-5-(hydroxymethyl)-2-methylisonicotinaldehyde 5-phosphate |
| 3-hydroxy-2-methyl-5-[(phosphonooxy)methyl]-4-pyridinecarboxaldehyde |
| Pyridoxal phosphate anhydrous |
| UNII-F06SGE49M6 |
| 2-Methyl-3-hydroxy-4-formyl-5-hydroxymethylpyridine-5-calcium phosphate |
| 4-pyridinecarboxaldehyde, 3-hydroxy-2-methyl-5-[(phosphonooxy)methyl]- |
| CHEMBL82202 |
| Vitamin B6 phosphate (ester) |
| F06SGE49M6 |
| CHEBI:18405 |
| pyridoxal-phosphate |
| Pyridoxal, 5-(dihydrogenphosphate) |
| NSC82388 |
| SRI 2392 |
| NSC-82388 |
| P-5'-P |
| MC-1 |
| Pyrido |
| [(4-formyl-5-hydroxy-6-methylpyridin-3-yl)methoxy]phosphonic acid |
| Pyridoxal, 5-(dihydrogen phosphate) (8CI) |
| (4-Formyl-5-hydroxy-6-methyl(3-pyridyl))methyl dihydrogen phosphate |
| 3-Hydroxy-2-methyl-5-([phosphonooxy]methyl)-4-pyridinecarboxaldehyde |
| Pyridoxal 5'-phosphate monohydrate, vitamin B6 |
| Vitamin B6 (Active form) |
| Pyridoxal 5 inverted exclamation marka-phosphate hydrate |
| 2-Methyl-3-hydroxy-4-formyl-5-hydroxymethylpyridine-5-calcium phosphate trihydrate |
| 2-Methyl-3-hydroxy-4-formyl-5-pyridylmethylphosphoric acid |
| 4-Formyl-5-hydroxy-6-methyl-pyridin-3-yl)methoxyphosphonic acid |
| Pyridoxal phosphate treated .beta.-lactoglobulin from bovine whey |
| (4-formyl-5-hydroxy-6-methyl-3-pyridinyl)methyl dihydrogen phosphate |
| (4-formyl-5-hydroxy-6-methyl-3-pyridyl)methyl dihydrogen phosphate |
| Pyridoxalphosphate |
| Phosphoric acid mono-(4-formyl-5-hydroxy-6-methyl-pyridin-3-ylmethyl) ester |
| Pyridoxal 5'-phosphate;Pyridoxyl phosphate |
| NCGC00166300-01 |
| Pyridoxal 5'-phosphate 5 |
| Lepirudine |
| C8H10NO6P |
| Pyridoxal-5-Phosphate Hydrate |
| Pyridoxal phosphate |
| Pridoxal-5-Phosphate |
| pyridoxal 5''-phosphate |
| 124051-94-1 |
| P5P |
| bmse000111 |
| D06JGH |
| D0U5YK |
| SCHEMBL23158 |
| Pyridoxal 5'-phosphoric acid |
| GTPL5249 |
| SGCUT00188 |
| DTXSID4048351 |
| Pyridoxal 5'-phosphate anhydrous |
| EX-A980 |
| BCP34576 |
| HY-B1744 |
| PYRIDOXAL 5-PHOSPHATE [MI] |
| to_000077 |
| BDBM50118216 |
| MFCD00006333 |
| PYRIDOXAL PHOSPHATE [WHO-DD] |
| s5311 |
| STL185213 |
| pyridoxal 5''-(dihydrogen phosphate) |
| PYRIDOXAL 5-PHOSPHATE [INCI] |
| AKOS015891654 |
| Pyridoxal 5'- (dihydrogen phosphate) |
| Pyridoxal 5'-phosphate;Codecarboxylase |
| CCG-266929 |
| CS-7767 |
| CS-O-06102 |
| DB00114 |
| SB18794 |
| PYRIDOXAL 5'-PHOSPHATE [VANDF] |
| Pyridoxal 5'-phosphate hydrate, >=98% |
| AS-19314 |
| Pyridoxal 5-phosphate;Pyridoxyl phosphate |
| LS-134383 |
| VITAMIN B6 (PYRIDOXAL 5-PHOSPHATE) |
| FT-0631236 |
| FT-0655876 |
| Isonicotinaldehyde, 5-(dihydrogen phosphate) |
| Pyridoxal 5'-phosphate monohydrate-Vitamin B6 |
| C00018 |
| F17391 |
| EN300-6474442 |
| Pyridoxal 5 inverted exclamation marka-phosphate |
| Pyridoxal 5'-phosphate monohydrate - Vitamin B6 |
| A841303 |
| Q418957 |
| SR-01000944534 |
| Q-201645 |
| SR-01000944534-1 |
| A26BDB6A-282A-4D13-A916-7B2B215B0FD6 |
| Z1741970251 |
| (4-Formyl-5-hydroxy-6-methylpyridin-3-yl)methyldihydrogenphosphate |
| 3-hydroxy-2-methyl-5-([phosphonooxy]methyl)-4-pyridinecarbaldehyde |
| (4-methanoyl-6-methyl-5-oxidanyl-pyridin-3-yl)methyl dihydrogen phosphate |
| 4-Pyridinecarboxaldehyde,3-hydroxy-2-methyl-5-[(phosphonooxy)methyl]- |
| 4-Pyridinecarboxaldehyde, 3-hydroxy-2-methyl-5-[(phosphonooxy)methyl]- (9CI) |
| 4-Pyridinecarboxaldehyde, 3-hydroxy-2-methyl-5-[(phosphonooxy)methyl]-(9CI) |
| Isonicotinaldehyde, 3-hydroxy-5-(hydroxymethyl)-2-methyl-, 5-(dihydrogen phosphate) |
| Pyridoxal 5'-phosphate hydrate, powder, BioReagent, suitable for cell culture |
|
There are more than 10 synonyms. If you wish to see them all click here.
|