| Medullin |
| PGA2 |
| 13345-50-1 |
| (+)-Prostaglandin A2 |
| 5,6-cis-PGA2 |
| (15S)-PGA2 |
| (+)-Prostaglandin A(sup 2) |
| K6VT5BDY9E |
| 15(S)-Prostaglandin A2 |
| CHEBI:27820 |
| Prosta-5,10,13-trien-1-oic acid, 15-hydroxy-9-oxo-, (5Z,13E,15S)- |
| 9-oxo-15S-hydroxy-5Z,10Z,13E-prostatrienoic acid |
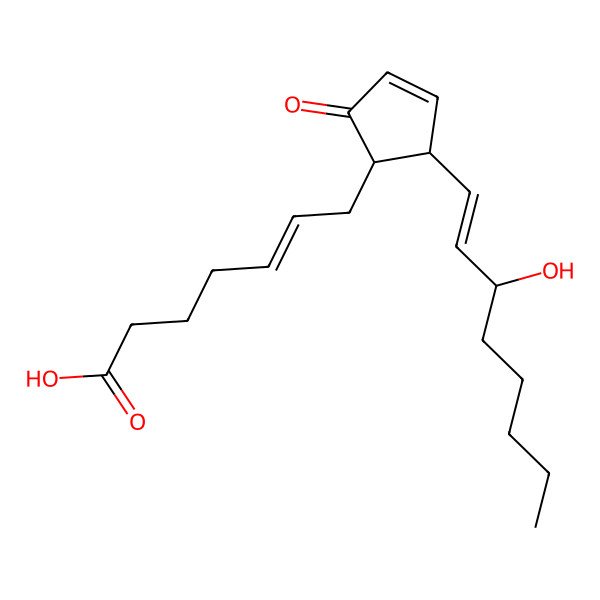
| (Z)-7-[(1R,2S)-2-[(E,3S)-3-hydroxyoct-1-enyl]-5-oxocyclopent-3-en-1-yl]hept-5-enoic acid |
| 15(S)-Hydroxy-9-oxo-5-cis-10,13-trans-prostatrienoic acid |
| Prosta-5,10,13-trien-1-oicacid, 15-hydroxy-9-oxo-, (5Z,13E,15S)- |
| NSC 165561 |
| PGA2 (Prostaglandin A2) |
| 15(S)-Hydroxy-9-oxo-5-cis-10,13-trans-prostenoic acid |
| PGA(sup 2) |
| 5,6-cis-PGA(sup 2) |
| MLS000069589 |
| Prostaglandin A(sup 2) |
| UNII-K6VT5BDY9E |
| (155)-PGA2 |
| BRN 2221844 |
| NSC-165561 |
| PGA2;Medullin |
| (~{Z})-7-[(1~{R},5~{S})-2-oxidanylidene-5-[(~{E},3~{S})-3-oxidanyloct-1-enyl]cyclopent-3-en-1-yl]hept-5-enoic acid |
| SMR000058790 |
| Pga2(prostaglandin a2) |
| BSPBio_001460 |
| BML1-F02 |
| GTPL6279 |
| SCHEMBL2115595 |
| CHEMBL1084643 |
| DTXSID20864388 |
| PROSTAGLANDIN A2 (PGA2) |
| HMS1361I22 |
| HMS1791I22 |
| HMS1989I22 |
| HMS3402I22 |
| HMS3648I11 |
| (5Z,13E,15S)-15-hydroxy-9-oxoprosta-5, 10,13-triene-1-oic acid |
| (5Z,13E,15S)-15-hydroxy-9-oxoprosta-5,10,13-trien-1-oic acid |
| LMFA03010035 |
| AKOS040755328 |
| Prosta-5,10-13-trien-1-oic acid, 15-hydroxy-9-oxo-, (5Z,13E,15S)- |
| (Z)-7-((1R,2S)-2-((E)-(3S)-3-hydroxyoct-1-enyl)-5-oxocyclopent-3-enyl)hept-5-enoic acid |
| 5-Heptenoic acid, 7-(2-(3-hydroxy-1-octenyl)-5-oxo-3-cyclopenten-1-yl)- (VAN) |
| IDI1_033930 |
| NCGC00022061-03 |
| NCGC00022061-04 |
| NCGC00022061-05 |
| HY-113041 |
| CS-0059420 |
| DINOPROSTONE IMPURITY D [EP IMPURITY] |
| C05953 |
| SR-01000946222 |
| SR-01000946222-1 |
| 15(S)-Hydroxy-9-oxo-5-cis-10,13-trans-prostenoate |
| BRD-K34782918-001-02-5 |
| Q27088462 |
| 15a-Hydroxy-9-oxo-cis-5,10,trans-13-prostatrienoate |
| 15(S)-Hydroxy-9-oxo-5-cis-10,13-trans-prostatrienoate |
| 15a-Hydroxy-9-oxo-cis-5,10,trans-13-prostatrienoic acid |
| 15a-Hydroxy-9-oxo-cis-5,10,trans-13-prostatrienecarboxylate |
| 362927F3-3A3E-47BC-84C3-C32A293AA956 |
| 15.alpha.-Hydroxy-9-oxo-cis-5,10,trans-13-prostatrienoic acid |
| 15a-Hydroxy-9-oxo-cis-5,10,trans-13-prostatrienecarboxylic acid |
| 15.alpha.-Hydroxy-9-oxo-cis-5,10,trans-13-prostatrienecarboxylic acid |
| (Z)-7-[(1R,2S)-2-[(E,3S)-3-hydroxyoct-1-enyl]-5-oxo-1-cyclopent-3-enyl]hept-5-enoic acid |
| (Z)-7-[(1R,2S)-2-[(E,3S)-3-hydroxyoct-1-enyl]-5-oxo-cyclopent-3-en-1-yl]hept-5-enoic acid |
| DINOPROSTONE IMPURITY, (5Z,13E,15S)-15-HYDROXY-9-OXOPROSTA-5, 10,13-TRIENE-1-OIC ACID- [USP IMPURITY] |
|
There are more than 10 synonyms. If you wish to see them all click here.
|