| 109-60-4 |
| N-PROPYL ACETATE |
| Acetic acid, propyl ester |
| Propyl ethanoate |
| 1-Acetoxypropane |
| 1-Propyl acetate |
| n-Propyl ethanoate |
| Octan propylu |
| Acetic acid n-propyl ester |
| Propylacetate |
| Acetate de propyle normal |
| n-Propyl acetate (natural) |
| Acetic acid propyl ester |
| FEMA No. 2925 |
| Propylester kyseliny octove |
| NSC 72025 |
| HSDB 161 |
| Octan propylu [Polish] |
| n-propanol acetate |
| EINECS 203-686-1 |
| Acetic acid, n-propyl ester |
| UNII-4AWM8C91G6 |
| BRN 1740764 |
| 4AWM8C91G6 |
| DTXSID6021901 |
| CHEBI:40116 |
| AI3-24156 |
| Acetate de propyle normal [French] |
| Propylester kyseliny octove [Czech] |
| NSC-72025 |
| UN1276 |
| DTXCID301901 |
| ACETIC ACID,PROPYL ESTER |
| EC 203-686-1 |
| 4-02-00-00138 (Beilstein Handbook Reference) |
| PROPYL ACETATE (USP-RS) |
| PROPYL ACETATE [USP-RS] |
| n-propylacetat |
| n-Propyl ester of acetic acid |
| ?Propyl acetate |
| acetic acid propyl |
| Propyl acetate, N- |
| ACETATE, PROPYL |
| Propyl acetate, 99% |
| PAT (CHRIS Code) |
| Actate de propyle normal |
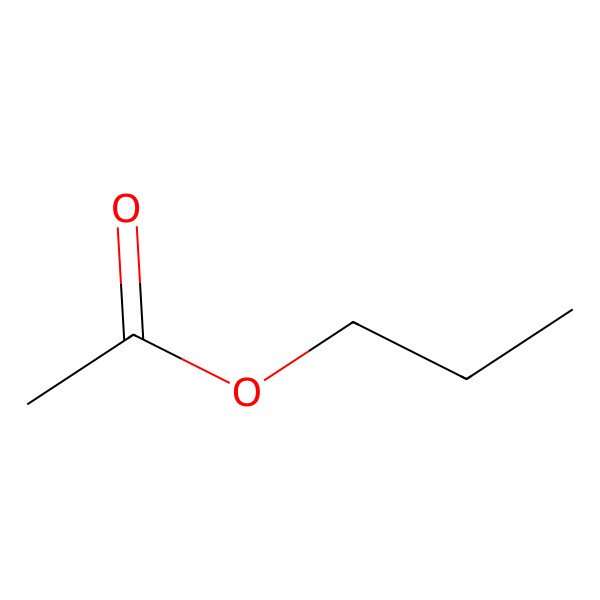
| CH3COOCH2CH2CH3 |
| Acetic acid-n-propyl ester |
| Propyl ester of acetic acid |
| PROPYL ACETATE [MI] |
| FEMA NUMBER 2935 |
| SCHEMBL14991 |
| PROPYL ACETATE [FCC] |
| WLN: 3OV1 |
| CHEMBL44857 |
| PROPYL ACETATE [FHFI] |
| PROPYL ACETATE [INCI] |
| Propyl acetate, >=99.5% |
| Propyl acetate, >=98%, FG |
| N-PROPYL ACETATE [HSDB] |
| N-Propyl acetate LBG-64752 |
| Propyl Acetate (Fragrance Grade) |
| Propyl Acetate (Industrial Grade) |
| Propyl acetate, analytical standard |
| ACETIC ACID, N-PROPYL ETHER |
| NSC72025 |
| Tox21_202012 |
| MFCD00009372 |
| NA1276 |
| STL280317 |
| AKOS008949448 |
| DB01670 |
| LS-3066 |
| UN 1276 |
| NCGC00249148-01 |
| NCGC00259561-01 |
| CAS-109-60-4 |
| A0044 |
| FT-0621756 |
| FT-0627474 |
| Propyl acetate, natural, >=97%, FCC, FG |
| n-Propyl acetate [UN1276] [Flammable liquid] |
| n-Propyl acetate [UN1276] [Flammable liquid] |
| Q415750 |
| J-002310 |
| InChI=1/C5H10O2/c1-3-4-7-5(2)6/h3-4H2,1-2H |
| Propyl acetate, United States Pharmacopeia (USP) Reference Standard |
| Propyl Acetate, Pharmaceutical Secondary Standard; Certified Reference Material |
|
There are more than 10 synonyms. If you wish to see them all click here.
|