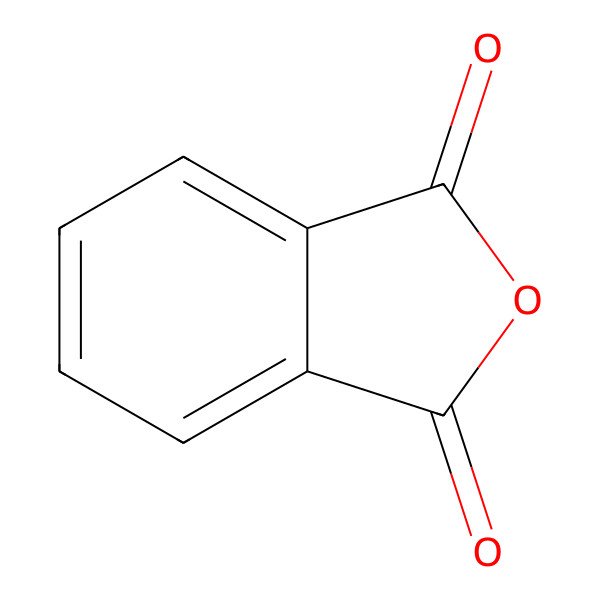
| 85-44-9 |
| Isobenzofuran-1,3-dione |
| 1,3-Isobenzofurandione |
| 2-Benzofuran-1,3-dione |
| 1,3-Dioxophthalan |
| 1,3-Phthalandione |
| Phthalsaeureanhydrid |
| o-Phthalic acid anhydride |
| Phthalic acid anhydride |
| Phthalandione |
| Retarder esen |
| Retarder AK |
| Retarder PD |
| Vulkalent B/C |
| Anidride ftalica |
| ESEN |
| Ftalowy bezwodnik |
| Ftaalzuuranhydride |
| Anhydride phtalique |
| Ftalanhydrid |
| 1,2-Benzenedicarboxylic anhydride |
| 1,2-Benzenedicarboxylic acid anhydride |
| Vulkalent B |
| Wiltrol P |
| Phthalanhydride |
| RCRA waste number U190 |
| NCI-C03601 |
| TGL 6525 |
| Anhydrid kyseliny ftalove |
| Sconoc 7 |
| Araldite HT 901 |
| Ftalanhydrid [Czech] |
| ortho-phthalic acid anhydride |
| CCRIS 519 |
| NSC 10431 |
| CHEBI:36605 |
| HSDB 4012 |
| Isobenzofuran, 1,3-dihydro-1,3-dioxo- |
| phtalic anhydride |
| Ftaalzuuranhydride [Dutch] |
| UNII-UVL263I5BJ |
| Anidride ftalica [Italian] |
| Ftalowy bezwodnik [Polish] |
| Retarder B-C |
| EINECS 201-607-5 |
| UVL263I5BJ |
| Anhydride phtalique [French] |
| 1,3-dihydro-2-benzofuran-1,3-dione |
| Phthalsaeureanhydrid [German] |
| DTXSID2021159 |
| AI3-04869 |
| Ht 901 |
| Anhydrid kyseliny ftalove [Czech] |
| PhthalicAcidAnhydride-d4 |
| MFCD00005918 |
| NSC-10431 |
| UN2214 |
| RCRA waste no. U190 |
| Phthalic anhydride (molten) |
| DTXCID401159 |
| EC 201-607-5 |
| 1,3-dihydro-1,3-dioxoisobenzofuran |
| 68411-80-3 |
| Ftalsyreanhydrid |
| C8H4O3 |
| pthalic anhydride |
| Retarder PX |
| Rikacid PA |
| 1,3-ftalandiona |
| Sconoc 5 |
| 1 3-Phthalandione |
| 1,3 Phthalandione |
| 1,3-isobenzofurandiona |
| 1,3 Isobenzofurandione |
| PAN (CHRIS Code) |
| Phthal ic acid anhydride |
| isobenzofurane-1,3-dione |
| 2-Benzofuran-1 3-dione |
| 2-Benzofuran-1,3-diona |
| SCHEMBL220 |
| Phthalic anhydride (8CI) |
| Epitope ID:112744 |
| WLN: T56 BVOVJ |
| 1,3-DIOXOPHTHALANE |
| 1,3-DIOXONAPHTHALAN |
| Isobenzo[b]furan-1,3-dione |
| 2-Benzofuran-1,3-dione # |
| UN 2214 (Salt/Mix) |
| Phthalic anhydride treated BSA |
| Phthalic anhydride treated HSA |
| Phthalic anhydride, ACS grade |
| PHTHALIC ANHYDRIDE [MI] |
| 12-Benzenedicarboxylic anhydride |
| CHEMBL1371297 |
| PHTHALIC ANHYDRIDE [HSDB] |
| PHTHALIC ANHYDRIDE [INCI] |
| Phthalic anhydride treated gelatin |
| Phthalic anhydride treated casein I |
| NSC10431 |
| EINECS 270-149-6 |
| Phthalic anhydride treated casein II |
| Tox21_200142 |
| 1,3-dihydroisobenzofuran-1,3-dione |
| Isobenzofuran,3-dihydro-1,3-dioxo- |
| NA2214 |
| STL194302 |
| AKOS000121309 |
| LS-1848 |
| CAS-85-44-9 |
| NCGC00091060-01 |
| NCGC00091060-02 |
| NCGC00257696-01 |
| BP-30002 |
| Phthalic anhydride, ACS reagent, >=99% |
| PS-10628 |
| Phthalic anhydride, for synthesis, 99.0% |
| Phthalic anhydride, ReagentPlus(R), 99% |
| 1,3-DIHYDRO-1,3-DIOXOISOBENZOFURANE |
| FT-0652549 |
| P1614 |
| Phthalic anhydride, purum, >=97.0% (NT) |
| EN300-18017 |
| Phthalic anhydride with >0.05% maleic anhydride |
| Phthalic anhydride, SAJ first grade, >=99.0% |
| A841333 |
| Q410882 |
| F1908-0105 |
| InChI=1/C8H4O3/c9-7-5-3-1-2-4-6(5)8(10)11-7/h1-4 |
| Phthalic anhydride with >0.05% maleic anhydride [UN2214] [Corrosive] |
| Phthalic anhydride with >0.05% maleic anhydride [UN2214] [Corrosive] |
| Phthalic anhydride, anhydrous, free-flowing, Redi-Dri(TM), ACS reagent, >=99% |
| 39363-63-8 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|