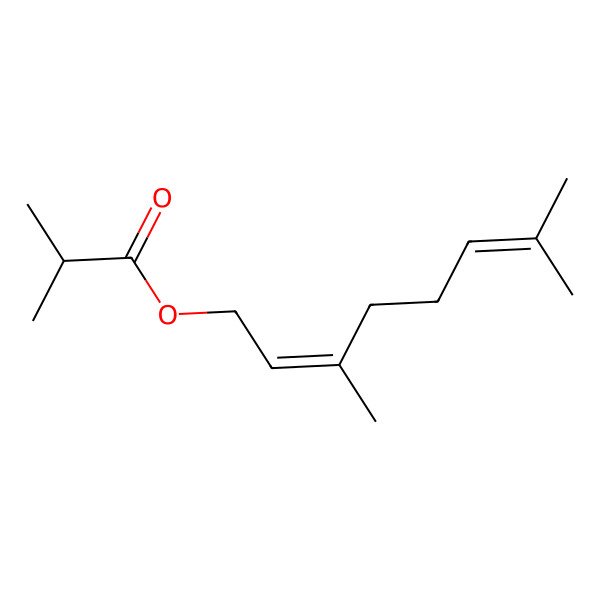
| 2345-24-6 |
| Geranyl 2-methylpropanoate |
| FEMA No. 2775 |
| Propanoic acid, 2-methyl-, (2Z)-3,7-dimethyl-2,6-octadienyl ester |
| Neryl isobutyrate (natural) |
| geranyl isobutanoate |
| Neryl 2-methylpropanoate, cis- |
| Propanoic acid, 2-methyl-, (2Z)-3,7-dimethyl-2,6-octadien-1-yl ester |
| EINECS 219-061-1 |
| cis-3,7-Dimethyl-2,6-octadien-1-yl isobutyrate |
| UNII-M9941BHL2O |
| M9941BHL2O |
| 3,7-Dimethyl-2,6-octadienyl isobutyrate, (Z)- |
| 3,7-Dimethyl-2,6-octadienyl 2-methylpropanoate, cis- |
| 3,7-Dimethyl-2,6-octadienyl 2-methylpropanoate, (Z)- |
| Isobutyric acid, 3,7-dimethyl-2,6-octadienyl ester, (Z)- |
| (Z)-2-Methylpropanoic acid 3,7-dimethyl-2,6-octadienyl ester |
| Propanoic acid, 2-methyl-, 3,7-dimethyl-2,6-octadienyl ester, (Z)- |
| (2Z)-3,7-dimethylocta-2,6-dien-1-yl 2-methylpropanoate |
| Isobutyric acid, (E)-3,7-dimethyl-2,6-octadienyl ester |
| Geraniol isobutyrate |
| 3,7-Dimethyl-2,6-octadienyl isobutyrate |
| Neryl iso-butyrate |
| geranyl iso-butyrate |
| neryl methylpropanoate |
| GERANYLISOBUTYRATE |
| 3,7-dimethylocta-2,6-dien-1-yl 2-methylpropanoate |
| Propanoic acid, 2-methyl-, (2E)-3,7-dimethyl-2,6-octadienyl ester |
| SCHEMBL1532475 |
| [(2Z)-3,7-dimethylocta-2,6-dienyl] 2-methylpropanoate |
| NERYL ISOBUTYRATE [FHFI] |
| FEMA 2513 |
| DTXSID30883828 |
| neryl isobutyrate, No Antioxidant |
| CHEBI:171784 |
| OGJYXQFXLSCKTP-LCYFTJDESA-N |
| LMFA07010895 |
| AKOS024319236 |
| AS-75605 |
| LS-121527 |
| Neryl isobutyrate, >=97%, stabilized, FG |
| CS-0449872 |
| (E)-3,7-Dimethyl-2,6-octadienyl isobutyrate |
| E79175 |
| (Z)-3,7-dimethylocta-2,6-dien-1-yl isobutyrate |
| 3,7-Dimethyl-isobutyratetrans-2,6-Octadien-1-ol |
| trans-3,7-dimethyl-2,6-octadien-1-yl isobutyrate |
| (2Z)-3,7-Dimethyl-2,6-octadienyl 2-methylpropanoate |
| (E)-3,7-Dimethyl-2,6-octadienyl 2-methylpropanoate |
| Q27283713 |
| (2E)-3,7-Dimethyl-2,6-octadienyl 2-methylpropanoate # |
| 3,7-Dimethyl-2,6-octadienyl estertrans-Isobutyric acid |
| Isobutyric acid (Z)-3,7-dimethyl-2,6-octadienyl ester |
| trans-3,7-Dimethyl-2,6-octadien-1-yl 2-methylpropanoate |
| Propanoic acid, 2-methyl-, 3,7-dimethyl-2,6-octadienyl ester, trans- |
| Propanoic acid,2-methyl-,(2Z)-3,7-dimethyl-2,6-octadien-1-yl ester |
| Propionic acid, 2-methyl-, 3,7-dimethyl-2,6-octadienyl ester, trans- |
|
There are more than 10 synonyms. If you wish to see them all click here.
|