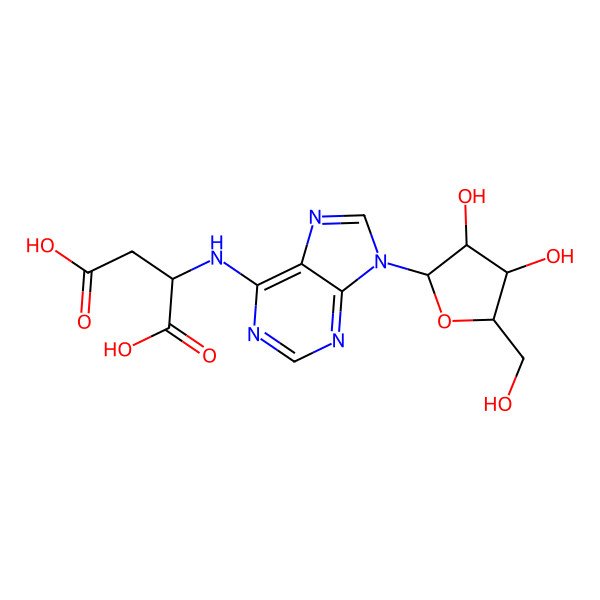
N-(9-Pentofuranosyl-9H-purin-6-yl)aspartic acid
| Internal ID | 7bf86dfb-8504-4d16-854a-cd867857bb6a |
| Taxonomy | Nucleosides, nucleotides, and analogues > Purine nucleosides |
| IUPAC Name | 2-[[9-[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]purin-6-yl]amino]butanedioic acid |
| SMILES (Canonical) | C1=NC(=C2C(=N1)N(C=N2)C3C(C(C(O3)CO)O)O)NC(CC(=O)O)C(=O)O |
| SMILES (Isomeric) | C1=NC(=C2C(=N1)N(C=N2)C3C(C(C(O3)CO)O)O)NC(CC(=O)O)C(=O)O |
| InChI | InChI=1S/C14H17N5O8/c20-2-6-9(23)10(24)13(27-6)19-4-17-8-11(15-3-16-12(8)19)18-5(14(25)26)1-7(21)22/h3-6,9-10,13,20,23-24H,1-2H2,(H,21,22)(H,25,26)(H,15,16,18) |
| InChI Key | VKGZCEJTCKHMRL-UHFFFAOYSA-N |
| Popularity | 28 references in papers |
| Molecular Formula | C14H17N5O8 |
| Molecular Weight | 383.31 g/mol |
| Exact Mass | 383.10771252 g/mol |
| Topological Polar Surface Area (TPSA) | 200.00 Ų |
| XlogP | -0.70 |
| Atomic LogP (AlogP) | -2.22 |
| H-Bond Acceptor | 11 |
| H-Bond Donor | 6 |
| Rotatable Bonds | 7 |
| SCHEMBL20099215 |
| DTXSID40863414 |
| FT-0674687 |
| N-(9-Pentofuranosyl-9H-purin-6-yl)aspartic acid |
| 2-[[9-[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]purin-6-yl]amino]butanedioic acid |
| Target | Value | Probability (raw) | Probability (%) |
|---|---|---|---|
| Human Intestinal Absorption | + | 0.6168 | 61.68% |
| Caco-2 | - | 0.9057 | 90.57% |
| Blood Brain Barrier | - | 0.5000 | 50.00% |
| Human oral bioavailability | - | 0.9000 | 90.00% |
| Subcellular localzation | Nucleus | 0.5118 | 51.18% |
| OATP2B1 inhibitior | - | 1.0000 | 100.00% |
| OATP1B1 inhibitior | + | 0.9263 | 92.63% |
| OATP1B3 inhibitior | + | 0.9456 | 94.56% |
| MATE1 inhibitior | - | 1.0000 | 100.00% |
| OCT2 inhibitior | - | 0.9861 | 98.61% |
| BSEP inhibitior | - | 0.8804 | 88.04% |
| P-glycoprotein inhibitior | - | 0.8549 | 85.49% |
| P-glycoprotein substrate | - | 0.8378 | 83.78% |
| CYP3A4 substrate | - | 0.5177 | 51.77% |
| CYP2C9 substrate | - | 0.6198 | 61.98% |
| CYP2D6 substrate | - | 0.8652 | 86.52% |
| CYP3A4 inhibition | - | 0.9710 | 97.10% |
| CYP2C9 inhibition | - | 0.9584 | 95.84% |
| CYP2C19 inhibition | - | 0.9674 | 96.74% |
| CYP2D6 inhibition | - | 0.9590 | 95.90% |
| CYP1A2 inhibition | - | 0.9411 | 94.11% |
| CYP2C8 inhibition | - | 0.8563 | 85.63% |
| CYP inhibitory promiscuity | - | 0.9726 | 97.26% |
| UGT catelyzed | + | 0.8000 | 80.00% |
| Carcinogenicity (binary) | - | 0.9200 | 92.00% |
| Carcinogenicity (trinary) | Non-required | 0.6291 | 62.91% |
| Eye corrosion | - | 0.9904 | 99.04% |
| Eye irritation | - | 0.9688 | 96.88% |
| Skin irritation | - | 0.7820 | 78.20% |
| Skin corrosion | - | 0.9428 | 94.28% |
| Ames mutagenesis | - | 0.5500 | 55.00% |
| Human Ether-a-go-go-Related Gene inhibition | - | 0.7712 | 77.12% |
| Micronuclear | + | 1.0000 | 100.00% |
| Hepatotoxicity | + | 0.5841 | 58.41% |
| skin sensitisation | - | 0.8743 | 87.43% |
| Respiratory toxicity | + | 0.7889 | 78.89% |
| Reproductive toxicity | + | 0.9667 | 96.67% |
| Mitochondrial toxicity | + | 0.9750 | 97.50% |
| Nephrotoxicity | - | 0.7400 | 74.00% |
| Acute Oral Toxicity (c) | III | 0.6259 | 62.59% |
| Estrogen receptor binding | - | 0.5398 | 53.98% |
| Androgen receptor binding | + | 0.6228 | 62.28% |
| Thyroid receptor binding | - | 0.5611 | 56.11% |
| Glucocorticoid receptor binding | - | 0.5465 | 54.65% |
| Aromatase binding | + | 0.6848 | 68.48% |
| PPAR gamma | + | 0.6476 | 64.76% |
| Honey bee toxicity | - | 0.8839 | 88.39% |
| Biodegradation | - | 0.7000 | 70.00% |
| Crustacea aquatic toxicity | - | 0.7200 | 72.00% |
| Fish aquatic toxicity | - | 0.7547 | 75.47% |
Proven Targets:
| CHEMBL ID | UniProt ID | Name | Min activity | Assay type | Source |
|---|---|---|---|---|---|
| No proven targets yet! | |||||
Predicted Targets (via Super-PRED):
| CHEMBL ID | UniProt ID | Name | Probability | Model accuracy |
|---|---|---|---|---|
| CHEMBL3251 | P19838 | Nuclear factor NF-kappa-B p105 subunit | 97.87% | 96.09% |
| CHEMBL3589 | P55263 | Adenosine kinase | 97.32% | 98.05% |
| CHEMBL226 | P30542 | Adenosine A1 receptor | 92.28% | 95.93% |
| CHEMBL221 | P23219 | Cyclooxygenase-1 | 91.38% | 90.17% |
| CHEMBL3060 | Q9Y345 | Glycine transporter 2 | 90.71% | 99.17% |
| CHEMBL2581 | P07339 | Cathepsin D | 90.36% | 98.95% |
| CHEMBL5619 | P27695 | DNA-(apurinic or apyrimidinic site) lyase | 89.46% | 91.11% |
| CHEMBL1075094 | Q16236 | Nuclear factor erythroid 2-related factor 2 | 87.92% | 96.00% |
| CHEMBL3776 | Q14790 | Caspase-8 | 87.89% | 97.06% |
| CHEMBL3038477 | P67870 | Casein kinase II alpha/beta | 87.18% | 99.23% |
| CHEMBL1075162 | Q13304 | Uracil nucleotide/cysteinyl leukotriene receptor | 86.37% | 80.33% |
| CHEMBL3137261 | O14744 | PRMT5/MEP50 complex | 84.37% | 100.00% |
| CHEMBL1806 | P11388 | DNA topoisomerase II alpha | 83.77% | 89.00% |
| CHEMBL1881 | P43116 | Prostanoid EP2 receptor | 81.35% | 93.00% |
| CHEMBL3401 | O75469 | Pregnane X receptor | 80.53% | 94.73% |
| PubChem | 14803430 |
| LOTUS | LTS0031850 |
| wikiData | Q105287736 |