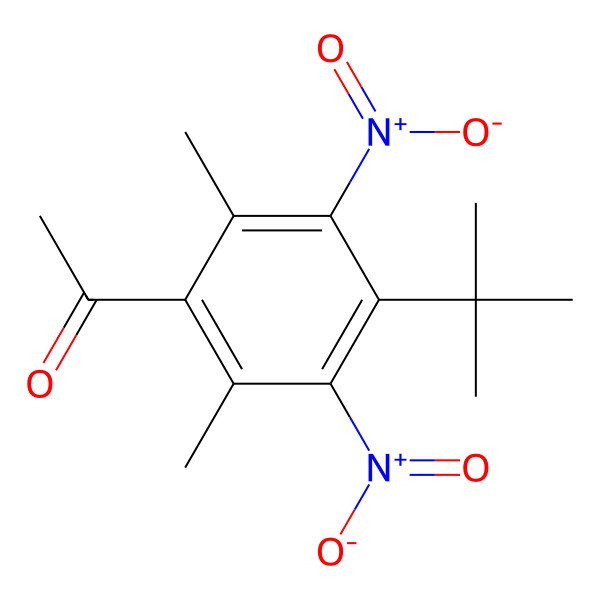
| 81-14-1 |
| 4'-tert-Butyl-2',6'-dimethyl-3',5'-dinitroacetophenone |
| Ketone moschus |
| 1-(4-tert-Butyl-2,6-dimethyl-3,5-dinitrophenyl)ethanone |
| Ketone musk |
| 2-Acetyl-5-tert-butyl-4,6-dinitroxylene |
| NSC 15339 |
| Acetophenone, 4'-tert-butyl-2',6'-dimethyl-3',5'-dinitro- |
| CCRIS 4677 |
| 3,5-Dinitro-2,6-dimethyl-4-tert-butylacetophenone |
| 4-tert-Butyl-2,6-dimethyl-3,5-dinitroacetophenone |
| 4-tert-Butyl-3,5-dinitro-2,6-dimethylacetophenone |
| 2,6-Dinitro-3,5-dimethyl-4-acetyl-tert-butylbenzene |
| C14H18N2O5 |
| EINECS 201-328-9 |
| 2,6-Dimethyl-3,5-dinitro-4-t-butylacetophenone |
| BRN 2062638 |
| UNII-483V3E1L6J |
| AI3-02440 |
| DTXSID6025690 |
| HSDB 7694 |
| 1-(4-(1,1-Dimethylethyl)-2,6-dimethyl-3,5-dinitrophenyl)ethanone |
| 483V3E1L6J |
| Ethanone, 1-(4-(1,1-dimethylethyl)-2,6-dimethyl-3,5-dinitrophenyl)- |
| NSC-15339 |
| Ethanone, 1-[4-(1,1-dimethylethyl)-2,6-dimethyl-3,5-dinitrophenyl]- |
| 4-t-Butyl-2,6-dimethyl-3,5-dinitroacetophenone |
| 4-07-00-00808 (Beilstein Handbook Reference) |
| 4-tert-Butyl-2,6-dimethyl-3,5-dinitroactophenone |
| 2,6-Dinitro-3,5-dimethyl-4-acetyl-tertbutylbenzene |
| 3,5-Dinitro-2,6-dimethyl-4-tert-butyl acetophenone |
| 1-Acetyl-4-tert-butyl-2,6-dimethyl-3,5-dinitrobenzene |
| 1-(4-tert-butyl-2,6-dimethyl-3,5-dinitrophenyl)ethan-1-one |
| Synthetic Musk ketone |
| Ketonmoschus |
| 1-[4-(1,1-Dimethylethyl)-2,6-dimethyl-3,5-dinitrophenyl]ethanone |
| MUSK, KETONE |
| Musk ketone, >=98% |
| Acetyl-dinitro-butyl-xylene |
| MUSK KETONE [HSDB] |
| MUSK KETONE [INCI] |
| SCHEMBL113330 |
| 2,6-Dimethyl-3,5-dinitro-4-tert-butylacetophenone |
| DTXCID405690 |
| CHEMBL1877463 |
| 1-(4-tert-butyl-2,6-dimethyl-3,5-dinitro-phenyl)ethanone |
| CHEBI:174791 |
| WXCMHFPAUCOJIG-UHFFFAOYSA-N |
| HY-N2045 |
| NSC15339 |
| Ketone Moschus, >=97.0% (N) |
| Tox21_301627 |
| BBL011671 |
| Dinitro-tert-butylxylyl methyl ketone |
| MFCD00024271 |
| STK725128 |
| AKOS005524284 |
| Musk ketone;3-Methylcyclopentadecanone |
| FS-4310 |
| LS-1807 |
| CAS-81-14-1 |
| Musk ketone 10 microg/mL in Cyclohexane |
| NCGC00164404-01 |
| NCGC00255386-01 |
| AC-11085 |
| AC-34811 |
| 4'-tert-Butyl-2',5'-dinitroacetophenone |
| Acetophenone,6'-dimethyl-3',5'-dinitro- |
| CS-0018535 |
| FT-0629036 |
| A840051 |
| SR-01000324027 |
| Q1948992 |
| SR-01000324027-1 |
| W-104216 |
| 2,6-dinitro-3,5-dimethyl-4-acetyl-tert-butyl benzene |
| 4'-tert-butyl-2',6'-dimethyl-3',5'-dinitroacetophenon |
| 2,6 DIMETHYL-4-TERT-BUTYL-3,5-DINITROACETOPHENONE |
| 2,6-DIMETHYL-3,5-DINITRO-4-TERT-BUTYL ACETOPHENONE |
| 3',5'-dinitro-2',6'-dimethyl-4'-tert-butylacetophenone |
| 3,5-DINITRO-4-TERT-BUTYL-2,6-DIMETHYLACETOPHENONE |
| 4'-tert-Butyl-2', 6'-dimethyl-3',5'-dinitroacetophenone |
| 4'-Tert-butyl-2',6'-dimethyl-3',5'-dinitro-Acetophenone |
| 4-ACETO-3,5-DIMETHYL-2,6-DINITRO-TERT-BUTYLBENZENE |
| 1-(4-tert-Butyl-2,6-dimethyl-3,5-dinitrophenyl)ethanone # |
| 1-TERT-BUTYL-3,5-DIMETHYL-2,6-DINITRO-4-ACETYLBENZENE |
| Ethanone,1-dimethylethyl)-2,6-dimethyl-3,5-dinitrophenyl]- |
| 1-[4-(tert-butyl)-2,6-dimethyl-3,5-dinitrophenyl]ethan-1-one |
| 4-tert-Butyl-2,6-dimethyl-3,5-dinitroacetophenone (Musk ketone) |
| 1-[4-(1,1-Dimethylethyl)-2,6-dimethyl-3,5-dinitrophenyl]ethanone, 9CI |
| 4' - tert - butyl - 2',6' - dimethyl - 3',5' - dinitroacetophenone |
| Ethanone, 1-[4-(1,1-dimethylethyl)- 2,6-dimethyl-3,5-dinitrophenyl]- |
|
There are more than 10 synonyms. If you wish to see them all click here.
|