| 1211-29-6 |
| (-)-Methyl jasmonate |
| Methyl cis-jasmonate |
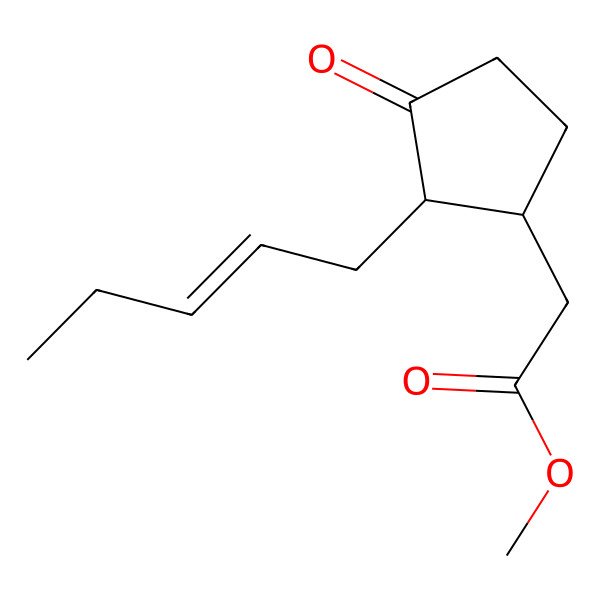
| Methyl 2-((1R,2R)-3-oxo-2-((Z)-pent-2-en-1-yl)cyclopentyl)acetate |
| methyl (-)-jasmonate |
| Jasmonic acid methyl ester |
| (-)-Jasmonic acid methyl ester |
| (3R,7R)-Methyl jasmonate |
| Methyl dl-jasmonate |
| Methyl jasmonic acid |
| CHEBI:15929 |
| Methyl (2-pent-2-enyl-3-oxo-1-cyclopentyl)acetate |
| 3-oxo-2-(2-pentenyl)cyclopentaneacetic acid methyl ester |
| UNII-900N171A0F |
| HSDB 8131 |
| methyl 2-[(1R,2R)-3-oxo-2-[(Z)-pent-2-enyl]cyclopentyl]acetate |
| Z-Methyl jasmonoate |
| EINECS 214-918-6 |
| EINECS 243-497-1 |
| Cyclopentaneacetic acid, 3-oxo-2-(2Z)-2-pentenyl-, methyl ester, (1R,2R)- |
| 900N171A0F |
| (-)-Jasmonic acid, methyl ester (trans) |
| Cyclopentaneacetic acid, 3-oxo-2-(2-pentenyl)-, methyl ester, (Z)-trans- |
| Methyl 3-oxo-2-(2-pentenyl)cyclopentaneacetate, (1R-(1alpha,2beta(Z)))- |
| Cyclopentaneacetic acid, 3-oxo-2-(2Z)-2-penten-1-yl-, methyl ester, (1R,2R)- |
| Methyljasmonate |
| methyl 2-((1R,2R)-3-oxo-2-pent-2Z-enyl)cyclopentyl)acetate |
| Methyl 3-oxo-2-(2-pentenyl)cyclopentaneacetate, (Z)-trans- |
| (+-)-Cyclopentaneacetic acid, 3-oxo-trans-2-(cis-2-pentenyl), methyl ester |
| Methyl (1R-(1alpha,2beta(Z)))-3-oxo-2-(pent-2-enyl)cyclopentaneacetate |
| Cyclopentaneacetic acid, 3-oxo-2-(2-pentenyl)-, methyl ester, (1R-(1alpha,2beta(Z)))- |
| Cyclopentaneacetic acid, 3-oxo-2-(2-pentenyl)-, methyl ester, (1theta-(1alpha,2beta(Z)))- |
| Cyclopentaneacetic acid, 3-oxo-trans-2-(cis-2-pentenyl), methyl ester |
| Methyl 3-oxo-2-(2-pentenyl)cyclopentaneacetate |
| 39924-52-2 |
| FEMA No. 3410 |
| Methyl [1R-[1alpha,2beta(Z)]]-3-oxo-2-(pent-2-enyl)cyclopentaneacetate |
| Cyclopentaneacetic acid, 3-oxo-2-(2-pentenyl)-, methyl ester, [1R-[1alpha,2beta(Z)]]- |
| Cyclopentaneacetic acid, 3-oxo-2-(2Z)-2-pentenyl-, methyl ester, (1R,2R)-rel- |
| 20073-13-6 |
| CYCLOPENTANEACETIC ACID, 3-OXO-2-(2-PENTENYL)-, METHYL ESTER, (1R-(1.ALPHA.,2.BETA.(Z)))- |
| Cyclopentaneacetic acid, 3-oxo-2-(2-pentenyl)-, methyl ester, [1R-[1.alpha.,2.beta.(Z)]]- |
| SCHEMBL36186 |
| METHYL JASMONATE [FHFI] |
| CHEMBL2139332 |
| DTXSID3036731 |
| Methyl [1alpha,2beta(Z)]-(+-)-3-oxo-2-(pent-2-enyl)cyclopentaneacetate |
| FEMA 3410 |
| (3R,7R)-(?)-Methyl jasmonate |
| CMC_7389 |
| Cyclopentaneacetic acid, 3-oxo-2-(2-pentenyl)-, methyl ester, [1.alpha.,2.beta.(Z)]-(.+-.)- |
| Cyclopentaneacetic acid, 3-oxo-2-(2-pentenyl)-, methyl ester, [1.alpha.,2.beta.(Z)]-(.+.)- |
| BDBM50509748 |
| CMC_13964 |
| LMFA02020010 |
| AKOS015950850 |
| JASMONIC ACID METHYL ESTER [MI] |
| AS-75742 |
| HY-135663 |
| CS-0113712 |
| D94878 |
| A891945 |
| Q26840883 |
| CYCLOPENTANEACETATE, 3-OXO-2-(2-PENTENYL)-, METHYL |
| 8171C3E0-B789-4211-B5C1-88C6B260AE3C |
| Cyclopentaneaceticacid,3-oxo-2-(2Z)-2-penten-1-yl-,methyl ester,(1R,2R)- |
| Methyl (1alpha,2beta(Z))-(1)-3-oxo-2-(pent-2-enyl)cyclopentaneacetate |
| methyl {(1R,2R)-3-oxo-2-[(2Z)-pent-2-en-1-yl]cyclopentyl}acetate |
| Cyclopentaneacetic acid, 3-oxo-2-(2-pentenyl)-, methyl ester, (Z)-trans- (8CI) |
| Cyclopentaneacetic acid, 3-oxo-2-(2Z)-2-penten-1-yl-, methyl ester, (1R,2R)-rel- |
| Cyclopentaneacetic acid, 3-oxo-2-(2Z)-2-pentenyl-, methyl ester, (1R,2R)- (9CI) |
| Cyclopentaneacetic acid, 3-oxo-2-[(2Z)-2-pentenyl]-, methyl ester, (1R,2R)- |
| Cyclopentaneaceticacid, 3-oxo-2-(2Z)-2-penten-1-yl-, methyl ester, (1R,2R)- |
| methyl (1R - (1a,2b(Z))) - 3 - oxo - 2 - (pent - 2 - enyl)cyclopentaneacetate |
|
There are more than 10 synonyms. If you wish to see them all click here.
|