| 110-27-0 |
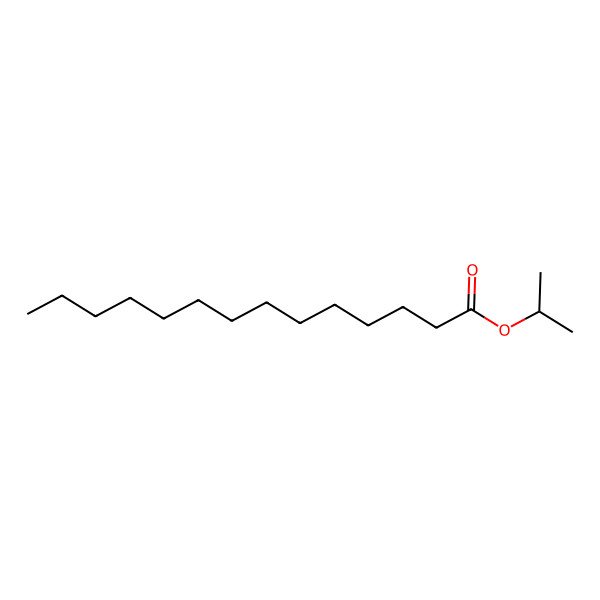
| Isopropyl tetradecanoate |
| Estergel |
| Isomyst |
| Bisomel |
| Promyr |
| Tetradecanoic acid, 1-methylethyl ester |
| Deltyl Extra |
| Kesscomir |
| Tegester |
| Sinnoester MIP |
| Crodamol IPM |
| Plymoutm IPM |
| Starfol IPM |
| Unimate IPM |
| Kessco IPM |
| Stepan D-50 |
| Emcol-IM |
| propan-2-yl tetradecanoate |
| Wickenol 101 |
| Emerest 2314 |
| 1-Methylethyl tetradecanoate |
| Deltylextra |
| Myristic acid isopropyl ester |
| JA-FA IPM |
| Crodamol I.P.M. |
| Kessco isopropyl myristate |
| FEMA No. 3556 |
| Tetradecanoic acid, isopropyl |
| Myristic acid, isopropyl ester |
| Tetradecanoic acid, isopropyl ester |
| Caswell No. 511E |
| HSDB 626 |
| NSC 406280 |
| Isopropyl myristate [USAN] |
| 1-Tridecanecarboxylic acid, isopropyl ester |
| UNII-0RE8K4LNJS |
| 0RE8K4LNJS |
| EINECS 203-751-4 |
| Estergel (TN) |
| EPA Pesticide Chemical Code 000207 |
| NSC-406280 |
| BRN 1781127 |
| methylethyl tetradecanoate |
| tetradecanoic acid 1-methylethyl ester |
| iso-Propyl N-tetradecanoate |
| Isopropyl myristate [USAN:NF] |
| DTXSID0026838 |
| CHEBI:90027 |
| EC 203-751-4 |
| Tetradecanoic acid methyethyl ester |
| 1405-98-7 |
| NCGC00164071-01 |
| WE(2:0(1Me)/14:0) |
| MYRISTIC ACID, ISOPROPYL ALCOHOL ESTER |
| Isopropyl myristate, 98% |
| TETRADECONOIC ACID, 1-METHYLETHYL ESTER |
| DTXCID306838 |
| ISOPROPYL MYRISTATE (II) |
| ISOPROPYL MYRISTATE [II] |
| ISOPROPYL MYRISTATE (MART.) |
| ISOPROPYL MYRISTATE [MART.] |
| ISOPROPYL MYRISTATE (USP-RS) |
| ISOPROPYL MYRISTATE [USP-RS] |
| CAS-110-27-0 |
| ISOPROPYL MYRISTATE (EP MONOGRAPH) |
| ISOPROPYL MYRISTATE [EP MONOGRAPH] |
| MFCD00008982 |
| Deltyextra |
| Tegosoft M |
| Liponate IPM |
| Crodamol 1PM |
| isopropyl-myristate |
| Lexol IPM |
| Crodamol I.P.M |
| isopropyl myristate |
| Isopropyltetradecanoate |
| myristic acid isopropyl |
| Radia 7190 |
| Isopropyl myristate (NF) |
| Isopropyl tetradecanoic acid |
| MYRISTATE, ISOPROPYL |
| SCHEMBL2442 |
| Myristic acid-isopropyl ester |
| Isopropyl myristate, >=98% |
| CHEMBL207602 |
| IPM 90 |
| ISOPROPYL MYRISTATE [MI] |
| WLN: 13VOY1&1 |
| FEMA 3556 |
| tetradecanoic acid isopropyl ester |
| ISOPROPYL MYRISTATE [FHFI] |
| ISOPROPYL MYRISTATE [HSDB] |
| ISOPROPYL MYRISTATE [INCI] |
| ISOPROPYL MYRISTATE [VANDF] |
| Isopropyl myristate, >=90% (GC) |
| Tox21_112080 |
| Tox21_202065 |
| Tox21_303171 |
| ISOPROPYL MYRISTATE [WHO-DD] |
| LMFA07010677 |
| NSC406280 |
| s2428 |
| AKOS015902296 |
| Tox21_112080_1 |
| DB13966 |
| LS-2869 |
| USEPA/OPP Pesticide Code: 000207 |
| NCGC00164071-02 |
| NCGC00164071-03 |
| NCGC00256937-01 |
| NCGC00259614-01 |
| HY-124190 |
| CS-0085813 |
| FT-0629053 |
| M0481 |
| D02296 |
| F71211 |
| EN300-25299830 |
| Q416222 |
| SR-01000944751 |
| Isopropyl myristate, Vetec(TM) reagent grade, 98% |
| Q-201418 |
| SR-01000944751-1 |
| Isopropyl myristate, United States Pharmacopeia (USP) Reference Standard |
| TETRADECANOIC ACID,ISOPROPYL ESTER (MYRISTATE,ISOPROPYL ESTER) |
| Isopropyl myristate, Pharmaceutical Secondary Standard; Certified Reference Material |
| Myristic acid, isopropyl ester; (Tetradecanoic acid, isopropyl; Isopropyl myristate) |
| InChI=1/C17H34O2/c1-4-5-6-7-8-9-10-11-12-13-14-15-17(18)19-16(2)3/h16H,4-15H2,1-3H |
|
There are more than 10 synonyms. If you wish to see them all click here.
|