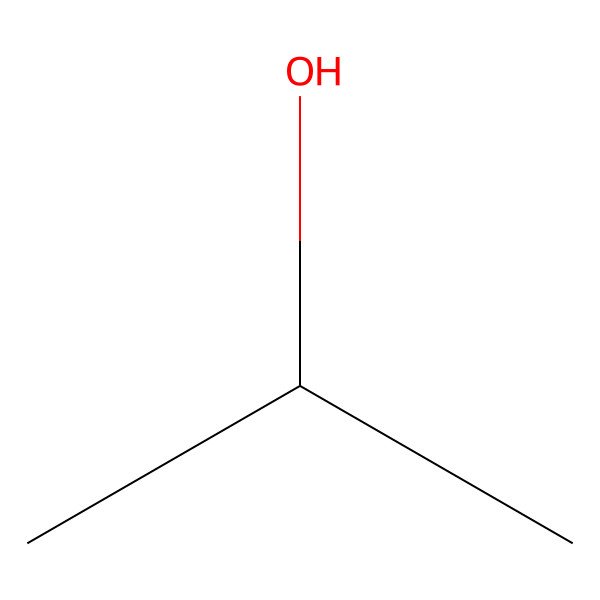
| isopropanol |
| 2-Propanol |
| Propan-2-ol |
| 67-63-0 |
| 2-Hydroxypropane |
| Alkolave |
| Avantine |
| Hartosol |
| Dimethylcarbinol |
| i-Propanol |
| sec-Propyl alcohol |
| Takineocol |
| Alcojel |
| Avantin |
| Petrohol |
| 1-Methylethanol |
| 2-Propyl alcohol |
| Isohol |
| Lavacol |
| Lutosol |
| Propol |
| Alcosolve 2 |
| Imsol A |
| Alcosolve |
| Arquad DMCB |
| Spectrar |
| 1-Methylethyl alcohol |
| Alcolo |
| i-Propylalkohol |
| Combi-schutz |
| n-Propan-2-ol |
| i-Propyl alcohol |
| Iso-propylalkohol |
| Isopropyl alcohol, rubbing |
| ISO-PROPANOL |
| Alcool isopropilico |
| Alcool isopropylique |
| Visco 1152 |
| sec-Propanol |
| Sterisol hand disinfectant |
| Iso-propyl alcohol |
| FEMA No. 2929 |
| i-Propanol [German] |
| Caswell No. 507 |
| iso-propylalcohol |
| Alcohol,isopropyl |
| FEMA Number 2929 |
| i-Propylalkohol [German] |
| Alcohol, Isopropyl |
| Iso-propylalkohol [German] |
| Alcool isopropilico [Italian] |
| Alcool isopropylique [French] |
| CCRIS 2308 |
| HSDB 116 |
| Virahol |
| Isopropylalkohol |
| 2-propanolum |
| dimethyl carbinol |
| propanol-2 |
| EINECS 200-661-7 |
| NSC 135801 |
| NSC-135801 |
| BRN 0635639 |
| ZURAGARD |
| DTXSID7020762 |
| UNII-ND2M416302 |
| CHEBI:17824 |
| AI3-01636 |
| Isopropyl alcohol [USP] |
| MFCD00011674 |
| UN1219 |
| Isopropyl alcohol [USAN] |
| ND2M416302 |
| IPS 1 |
| DTXCID50762 |
| EC 200-661-7 |
| 4-01-00-01461 (Beilstein Handbook Reference) |
| NSC135801 |
| ISOPROPYL ALCOHOL (ISOPROPANOL) |
| 2-Propanol, anhydrous |
| NCGC00090917-01 |
| (component of) Hibistat |
| Rubbing alcohol |
| DURAPREP COMPONENT ISOPROPYL ALCOHOL |
| SOLUPREP COMPONENT ISOPROPYL ALCOHOL |
| Isopropyl alcohol (USP) |
| CHLORAPREP COMPONENT ISOPROPYL ALCOHOL |
| ISOPROPYL ALCOHOL COMPONENT OF DURAPREP |
| ISOPROPYL ALCOHOL COMPONENT OF SOLUPREP |
| iPrOH |
| ISOPROPYL ALCOHOL COMPONENT OF CHLORAPREP |
| Isopropyl alcohol (manufacture strong-acid process) |
| ISOPROPYL ALCOHOL (II) |
| ISOPROPYL ALCOHOL [II] |
| ISOPROPYL ALCOHOL (IARC) |
| ISOPROPYL ALCOHOL [IARC] |
| 2-Propanol, USP, 99.0% |
| Isopropyl alcohol (manufacturing-strong acid process only) |
| ISOPROPYL ALCOHOL (MART.) |
| ISOPROPYL ALCOHOL [MART.] |
| isopropylalcohol |
| ISOPROPYL ALCOHOL(2-D) |
| 2 Propanol |
| 2-Propanol, ACS reagent, >=99.5% |
| CAS-67-63-0 |
| ISOPROPYL ALCOHOL (EP MONOGRAPH) |
| ISOPROPYL ALCOHOL [EP MONOGRAPH] |
| ACETONE IMPURITY B (EP IMPURITY) |
| ACETONE IMPURITY B [EP IMPURITY] |
| ISOPROPYL ALCOHOL (USP MONOGRAPH) |
| ISOPROPYL ALCOHOL [USP MONOGRAPH] |
| Isopropyl alcohol (only persons who manufacture by the strong acid process are subject, supplier notification not required) |
| 21388-65-8 |
| 84809-71-2 |
| ISOPROPYL ALCOHOL(1,1,1,3,3,3-D6) |
| ISOPROPYL ALCOHOL(1,1,1,2,3,3,3-D7) |
| isoproylalcohol |
| i-propylalcohol |
| Isopro |
| Lavaco |
| 2propanol |
| 2-propylalcohol |
| isopropy alcohol |
| isoproyl alcohol |
| 2-PROPANOL, ISOPROPANOL |
| Isopropyl-alcohol |
| propane-2ol |
| propan-2ol |
| 2-propano |
| Isopropryl alcohol |
| propane-2-ol |
| 2 -propanol |
| 2- propanol |
| propan 2-ol |
| iso-PrOH |
| Isopropyl Alcohol |
| Alcohol isopropilico |
| i-PrOH |
| (propan-2-ol) |
| Isopro (TN) |
| Recovered Isopropanol |
| AUTOSEPT |
| Tissue Dry Dehydrant |
| Caswell No 507 |
| IPS 1 (alcohol) |
| Propan- 2- ol |
| Isopropyl Alcohol C+ |
| Hibistat (Salt/Mix) |
| i-Pr-OH |
| Propane, 2-hydroxy- |
| 2-Propanol, Ph Eur |
| iso-C3H7OH |
| Isopropanol (JP17) |
| propan - 2 - ol |
| IPA (CHRIS Code) |
| Isopropanol (Recovered) |
| Isopropanol ACS reagent |
| 2-Propanol, GC Grade |
| 2-Propanol, for HPLC |
| ISOPROPANOL [JAN] |
| Isopropyl Alcohol - GMP |
| 2-Propanol, ACS reagent |
| 2-Propanol, HPLC Grade |
| CHEMBL582 |
| D03AAP |
| D0W7LK |
| Isopropyl alcohol HPLC/UV |
| 2-Propanol, 99.5% |
| Isopropanol, 70% in water |
| Isopropanol, 70% in H2O |
| ISOPROPANOL [WHO-DD] |
| 2-PROPANOL [USP-RS] |
| 2-PROPANOL [WHO-IP] |
| Isopropanol, Isopropyl Alcohol |
| ISOPROPYL ALCOHOL [MI] |
| SECONDARY PROPYL ALCOHOL |
| C(C)(C)O |
| isopropanol (isopropyl alcohol) |
| Isopropanol, 99.5% anhydrous |
| WLN: QY1&1 |
| 2-Propanol, analytical standard |
| Isopropanol or isopropyl alcohol |
| ISOPROPYL ALCOHOL [FCC] |
| 2-Propanol, LR, >=99% |
| ISOPROPYL ALCOHOL [FHFI] |
| ISOPROPYL ALCOHOL [HSDB] |
| ISOPROPYL ALCOHOL [INCI] |
| BDBM36154 |
| ALCOHOL,ISOPROPYL [VANDF] |
| ISOPROPYL ALCOHOL [VANDF] |
| 2-Propanol, anhydrous, 99.5% |
| 2-Propanol, AR, >=99.5% |
| AMY11029 |
| STR00089 |
| 2-Propanol, 99.9%, PRA grade |
| 2-Propanol, for HPLC, 99.5% |
| 2-Propanol, for HPLC, 99.9% |
| 2-Propanol, technical grade, 95% |
| Isopropyl Alcohol Reagent Grade ACS |
| Tox21_111039 |
| Tox21_202475 |
| 2-Propanol, 99.5%, HPLC grade |
| 2-PROPANOLUM [WHO-IP LATIN] |
| NA1219 |
| 2-Propanol, histological grade, 99% |
| AKOS000121012 |
| 2-Propanol, for HPLC, >=99.8% |
| DB02325 |
| EPA Pesticide Chemical Code 047501 |
| ISOPROPYL ALCOHOL [ORANGE BOOK] |
| LS-1565 |
| UN 1219 |
| USEPA/OPP Pesticide Code: 047501 |
| NCGC00260024-01 |
| 2-Propanol, 99.95% (LC-MS Grade) |
| 2-Propanol, UV HPLC gradient, 99.8% |
| Isopropyl alcohol, natural, >=98%, FG |
| 2-Propanol 100 microg/mL in Acetonitrile |
| Isopropyl alcohol, >=99.7%, FCC, FG |
| 2-Propanol, SAJ first grade, >=99.0% |
| 2-Propanol, JIS special grade, >=99.5% |
| 2-Propanol, Laboratory Reagent, >=99.5% |
| 2-Propanol, meets USP testing specifications |
| FT-0613371 |
| FT-0670483 |
| I0163 |
| I0164 |
| I0277 |
| EN300-21633 |
| Isopropanol, 99.5% anhydrous, 50 ppm water |
| C01845 |
| D00137 |
| Q16392 |
| R00276 |
| InChI=1/C3H8O/c1-3(2)4/h3-4H,1-2H |
| SR-01000944474 |
| ISOPROPYL ALCOHOL (MFG-STRONG ACID PROCESS) |
| J-510285 |
| SR-01000944474-1 |
| 2-Propanol, ACS spectrophotometric grade, >=99.5% |
| 2-Propanol, p.a., ACS reagent, reag. ISO, 99.8% |
| 2-Propanol, reag. ISO, UV HPLC spectroscopic, 99.7% |
| 2-Propanol, BioReagent, for molecular biology, >=99.5% |
| 2-Propanol, electronic grade, 99.999% trace metals basis |
| 2-Propanol, puriss. p.a., ACS reagent, >=99.8% (GC) |
| 2-Propanol, Vetec(TM) reagent grade, anhydrous, >=99.5% |
| 2-Propanol, BioUltra, for molecular biology, >=99.5% (GC) |
| Isopropanol or isopropyl alcohol [UN1219] [Flammable liquid] |
| Isopropanol or isopropyl alcohol [UN1219] [Flammable liquid] |
| Isopropyl alcohol, EuropePharmacopoeia (EP) Reference Standard |
| Isopropyl alcohol, meets EP, BP, USP testing specifications |
| 2-Propanol, >=99.7%, suitable for absorption spectrum analysis |
| 2-Propanol, semiconductor grade PURANAL(TM) (Honeywell 17829) |
| 2-Propanol, United States Pharmacopeia (USP) Reference Standard |
| 2-Propanol, Pharmaceutical Secondary Standard; Certified Reference Material |
| 2-Propanol, semiconductor grade MOS PURANAL(TM) (Honeywell 17930) |
| 2-Propanol, semiconductor grade SLSI PURANAL(TM) (Honeywell 17030) |
| 2-Propanol, semiconductor grade ULSI PURANAL(TM) (Honeywell 17022) |
| 2-Propanol, semiconductor grade VLSI PURANAL(TM) (Honeywell 17604) |
| 2-Propanol, puriss. p.a., ACS reagent, reag. ISO, reag. Ph. Eur., >=99.8% (GC) |
| 2-Propanol, puriss., meets analytical specification of Ph. Eur., BP, USP, >=99.5% (GC) |
| 8003-15-4 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|