| 133-32-4 |
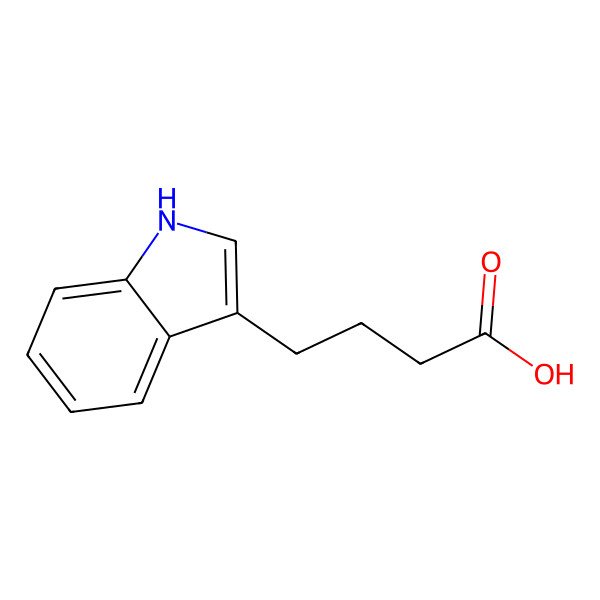
| 3-Indolebutyric acid |
| 4-(1H-Indol-3-yl)butanoic acid |
| Indolebutyric acid |
| Hormodin |
| 1H-INDOLE-3-BUTANOIC ACID |
| Seradix |
| Indole-3-butanoic acid |
| Jiffy grow |
| 4-(3-Indolyl)butyric acid |
| 4-indol-3-ylbutyric acid |
| IBA |
| Indolbutyric acid |
| 4-(Indol-3-yl)butyric acid |
| 1H-Indole-3-butyric acid |
| Seradix 2 |
| Seradix 3 |
| Seradix B 2 |
| Seradix B 3 |
| Rhizopon AA |
| beta-Indolebutyric acid |
| Butyric acid, 4-(indolyl)- |
| Indol-3,4'-yl butyric acid |
| 3-Indolylbutyric acid |
| Caswell No. 499 |
| 3-Indolebutyric acid (IBA) |
| 3-Indolebutyricacid |
| 4-(3-Indolyl)butanoic acid |
| 3-Indolyl-gamma-butyric acid |
| CCRIS 1020 |
| Indolyl-3-butyric acid |
| 933-32-4 |
| HSDB 7214 |
| 3-Indolebutyrate |
| NSC 3130 |
| EPA Pesticide Chemical Code 046701 |
| .beta.-IBA |
| CHEBI:33070 |
| 3-Indole butyric acid |
| AI3-17434 |
| 4-(1H-Indol-3-yl)butyric acid |
| 4-(1H-Indol-3-yl)-butyric acid |
| .beta.-Indolebutyric acid |
| 061SKE27JP |
| 3-Indolyl-.gamma.-butyric acid |
| DTXSID8032623 |
| .gamma.-(3-Indolyl)butyric acid |
| .gamma.-(Indole-3)-butyric acid |
| .gamma.-(Indol-3-yl)butyric acid |
| NSC-3130 |
| 1H-Indole-3-butyrate |
| beta-Indolylbutyric acid |
| 1H-Indole-3-butanoate |
| 4-(3-Indolyl)butyric acid 100 microg/mL in Acetonitrile |
| gamma-(Indol-3-Yl)butyric acid |
| 3IB |
| Indole 3-butyric acid |
| SR-01000477768 |
| EINECS 205-101-5 |
| 4-indol-3-ylbutanoic acid |
| 4-Indol-3-ylbutyric acid [BSI:ISO] |
| BRN 0171120 |
| Kyselina 4-indol-3-ylmaselina [Czech] |
| UNII-061SKE27JP |
| Indolebutyrate |
| Chryzopon |
| Chryzosan |
| Chryzotek |
| Oxyberon |
| Hormex |
| Kyselina 4-indol-3-ylmaselina |
| b-Indolebutyrate |
| 3-Iodolebutyrate |
| 3-Indole butyrate |
| beta-Indolebutyrate |
| Indole 3-butyrate |
| Indole-3 Butyrate |
| Indolyl-3-butyrate |
| 1H-Indole-3-butanoic acid (9CI) |
| Indole-3-Butrylate |
| b-Indolebutyric acid |
| 3-indolebutyric-acid |
| MFCD00005664 |
| 4-indol-3-ylbutyrate |
| Indole-3 Butyric acid |
| Indole-3-Butrylic acid |
| 4-(indolyl)- butyrate |
| Enamine_005659 |
| 4-(3-Indolyl)butyrate |
| 4-(3-Indole)-butyrate |
| 4-(Indol-3-yl)butyrate |
| .gamma.-Indolebutyric acid |
| 4-Indol-3-ylbutyric-acid |
| CBKinase1_000084 |
| CBKinase1_012484 |
| 4-(3-Indole)butyric acid |
| 4-(indolyl)- butyric acid |
| 4-(Indol-3)-butyric acid |
| 4-(3-1H-Indolyl)butyrate |
| Oprea1_093618 |
| Oprea1_136608 |
| SCHEMBL35884 |
| 3-Indolebutyric acid, 98% |
| 4-(3-Indole)-butyric acid |
| CBDivE_001082 |
| 5-22-03-00140 (Beilstein Handbook Reference) |
| MLS006011821 |
| 4-(3-Indolyl)-butyric acid |
| WLN: T56 BMJ D3VQ |
| .gamma.-Indole-3-butyric acid |
| CHEMBL582878 |
| gamma-(Indole-3)-butyric acid |
| Indole-3-butyric acid (8CI) |
| SCHEMBL1087345 |
| INDOLEBUTYRIC ACID [MI] |
| 4-(1H-Indol-3-yl)-butyrate |
| 4-(3-1H-Indolyl)butyric acid |
| DTXCID6012623 |
| HMS1410B05 |
| HMS3604N19 |
| HMS3655J16 |
| BCP00909 |
| HY-N0186 |
| STR04308 |
| Tox21_301287 |
| BDBM50129081 |
| s2253 |
| 4-(1H-Indol-3-yl)butanoic acid # |
| [3-(3-Indolyl)propyl]carboxylic acid |
| AKOS000273851 |
| AC-2855 |
| BCP9000128 |
| CCG-110315 |
| CG-0518 |
| CS-6288 |
| DB02740 |
| SB15075 |
| IDI1_007894 |
| 4-INDOL-3-YLBUTYRIC ACID [ISO] |
| NCGC00164296-01 |
| NCGC00164296-02 |
| NCGC00164296-03 |
| NCGC00164296-05 |
| NCGC00257530-01 |
| 4-INDOL-3-YLBUTYRIC ACID [HSDB] |
| CAS-133-32-4 |
| SMR001252209 |
| Indole-3-butyric acid, >=99.0% (T) |
| AM20060321 |
| BB 0242435 |
| FT-0602391 |
| FT-0691186 |
| I0026 |
| SW219851-1 |
| EN300-16684 |
| A15199 |
| D78109 |
| I-2000 |
| I-2005 |
| I-2010 |
| AB00375585-03 |
| Q2622539 |
| SR-01000477768-1 |
| SR-01000477768-3 |
| BRD-K43187796-237-01-5 |
| Indole-3-butyric acid, Vetec(TM) reagent grade, 98% |
| Z56754864 |
| F0919-1266 |
| Indole-3-butyric acid, PESTANAL(R), analytical standard |
| 67085FE2-19DF-4524-B354-17BD9781B318 |
| Indole-3-butyric acid, plant cell culture tested, BioReagent |
|
There are more than 10 synonyms. If you wish to see them all click here.
|