| 482-89-3 |
| Indigotin |
| Indigo Blue |
| Vat blue 1 |
| Cystoceva |
| Diindogen |
| Vulcafix Blue R |
| Indigo Pure BASF |
| Indigo Ciba |
| Indigo Synthetic |
| Synthetic Indigo |
| Indigo J |
| Indigo N |
| Indigo P |
| Indigo Powder W |
| Vulcafor Blue A |
| Vynamon Blue A |
| Indigo VS |
| Indigo Ciba SL |
| Indigo NAC |
| Indigo PLN |
| Indigo NACCO |
| C.I. Vat Blue 1 |
| Indigo (synthetic) |
| Mitsui Indigo Pure |
| Mitsui Indigo Paste |
| indigo dye |
| Synthetic Indigo TS |
| Vulcol Fast Blue GL |
| Lithosol Deep Blue B |
| Vulcanosine Dark Blue L |
| D&C Blue No. 6 |
| Blue No. 201 |
| D and C Blue No. 6 |
| Lithosol deep blue V |
| Indigo,natural |
| Indigo Pure BASF Powder K |
| 11669 Blue |
| Monolite Fast Navy Blue BV |
| Modr kypova 1 |
| CI 73000 |
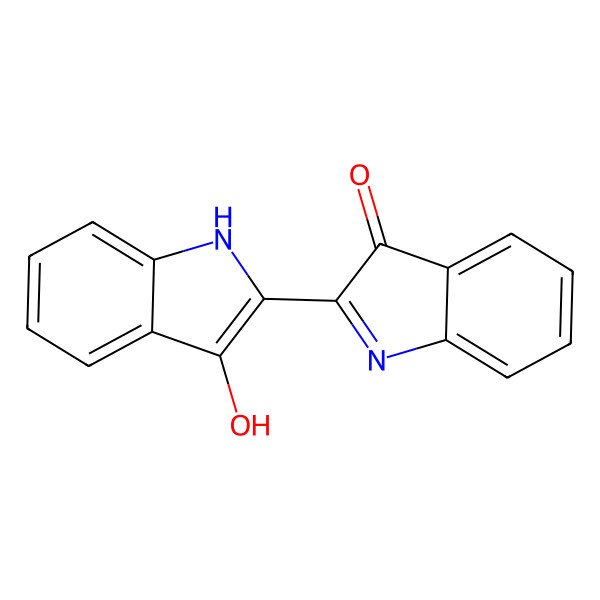
| 3H-Indol-3-one, 2-(1,3-dihydro-3-oxo-2H-indol-2-ylidene)-1,2-dihydro- |
| C.I. 73000 |
| Indigo Naturalis |
| NCI-C61392 |
| (2,2'-Biindoline)-3,3'-dione |
| 12626-73-2 |
| 68651-46-7 |
| Indigo [HPUS] |
| Indigo [HSDB] |
| Indigo [MI] |
| indigo blue (powder) |
| C.I Vat blue 1 |
| AO201 |
| D&C Blue No.6 |
| D&C Blue 6 |
| D+C Blue No. 6 |
| NSC 8645 |
| NSC-8645 |
| MFCD00005722 |
| D And C Blue Number 6 |
| 2-(1,3-Dihydro-3-oxo-2H-indol-2-ylidene)-1,2-dihydro-3H-indol-3-one |
| (2E)-2-(3-oxo-1H-indol-2-ylidene)-1H-indol-3-one |
| C.I.73000 |
| D&C Blue No. 6 [II] |
| 1G5BK41P4F |
| AO201 [INCI] |
| CHEMBL599552 |
| .DELTA.2,2'-Bipseudoindoxyl |
| 2-(3-hydroxy-1H-indol-2-yl)indol-3-one |
| DTXSID3026279 |
| CI 73000 [INCI] |
| [2,2'-Biindolinylidene]-3,3'-dione |
| NCGC00163355-01 |
| 2,2'-Biindole-3,3'(1H,1'H)-dione |
| (delta(sup 2,2')-Biindoline)-3,3'-dione |
| (Z)-[2,2'-Biindolinylidene]-3,3'-dione |
| 1H,1'H-[2,2']Biindolylidene-3,3'-dione |
| 64784-13-0 |
| Indigo (VAN) |
| CI Vat Blue 1 |
| DTXCID101022739 |
| Modr Kypova 1 [Czech] |
| delta2,2'-Bipseudoindoxyl |
| D & C Blue No. 6 |
| Indigo (dye) |
| 2001554-70-5 |
| CAS-482-89-3 |
| SMR000857361 |
| CCRIS 4379 |
| HSDB 4372 |
| (2E)-2,2'-biindole-3,3'(1H,1'H)-dione |
| delta(sup 2,2')-Bipseudoindoxyl |
| EINECS 207-586-9 |
| BRN 0088275 |
| UNII-1G5BK41P4F |
| Indigoblau |
| cis-indigo |
| AI3-09080 |
| indigo B |
| Indigo, synthetic |
| (delta2,2'(3H,3'H)-Biindole)-3,3'-dione |
| Indigotin (natural) |
| Indigo,(S) |
| (delta2,2'-Biindoline)-3,3'-dione |
| D & C blue No 6 |
| INDIGO [WHO-DD] |
| 2-(1,3-Dihydro-3-oxo-2H-indazol-2-ylidene)-1,2-dihydro-3H-indol-3-one |
| EC 207-586-9 |
| [.DELTA.2,3'-dione |
| (2E)-2-(3-oxoindolin-2-ylidene)indolin-3-one |
| SCHEMBL42280 |
| SCHEMBL56085 |
| 5-24-08-00503 (Beilstein Handbook Reference) |
| MLS001335921 |
| MLS001335922 |
| CHEMBL35479 |
| MEGxp0_001924 |
| Indigo (C.I. 73000) |
| SCHEMBL23064865 |
| ACon1_002192 |
| COHYTHOBJLSHDF-BUHFOSPRSA- |
| NSC8645 |
| CHEBI:177687 |
| COHYTHOBJLSHDF-BUHFOSPRSA-N |
| BCP26169 |
| HY-N0335 |
| Tox21_112052 |
| Tox21_202545 |
| BDBM50310357 |
| s3876 |
| 2-(1,3-Dihydro-3-oxo-2H-indol-2-ylidene)-1,2-dihydro-3H-indol-3-o- ne |
| AKOS015900171 |
| AKOS015955899 |
| Indigo, synthetic, Dye content 95 % |
| Tox21_112052_1 |
| AC-8002 |
| CCG-267072 |
| CID 5354391 |
| NCGC00091633-01 |
| NCGC00163355-02 |
| NCGC00163355-03 |
| NCGC00163355-04 |
| NCGC00163355-05 |
| NCGC00260094-01 |
| PD100504 |
| SY108978 |
| [(D)(2,2')-Biindoline]-3,3'-dione |
| 2-(3-oxoindolin-2-ylidene)indolin-3-one |
| (.DELTA.2,2'-Biindoline)-3,3'-dione |
| (E)-[2,2'-Biindolinylidene]-3,3'-dione |
| [.DELTA.2,3'H)-Biindole]-3,3'-dione |
| CS-0008896 |
| FT-0627197 |
| I0212 |
| VAT BLUE 1 (INDIGO C.I. 73000) |
| Q422662 |
| SR-01000941903 |
| (.DELTA.2,2'(3H,3'H)-Biindole)-3,3'-dione |
| Indigo, EuropePharmacopoeia (EP) Reference Standard |
| J-521528 |
| SR-01000941903-2 |
| (.DELTA.(SUP 2,2')-BIINDOLINE)-3,3'-DIONE |
| 3-CARBOXY-1,4-DIMETHYL-1H-PYRROLE-2-ACETICACID |
| 2-(3-oxo-2,3-dihydro-1h-indol-2-ylidene)-2,3-dihydro-1h-indol-3-one |
| (2,2 inverted exclamation mark -biindoline)-3,3 inverted exclamation mark -dione |
| InChI=1/C16H10N2O2/c19-15-9-5-1-3-7-11(9)17-13(15)14-16(20)10-6-2-4-8-12(10)18-14/h1-8,17-18H/b14-13+ |
|
There are more than 10 synonyms. If you wish to see them all click here.
|