| 630-56-8 |
| Delalutin |
| Primolut Depot |
| Hydroxyprogesterone hexanoate |
| Hormofort |
| Proge |
| Syngynon |
| Depo-Proluton |
| Proluton Depot |
| Hylutin |
| Teralutil |
| Makena |
| Estralutin |
| Hyproval |
| Kaprogest |
| Lutate |
| Relutin |
| Progesterone caproate |
| Neolutin forte |
| 17-Caproxyprogesterone |
| Idrogestene |
| Duraluton |
| Hyroxon |
| Luteocrin |
| Lutopron |
| Luteocrin depot |
| Hyproval-PA |
| 17alpha-Hydroxyprogesterone caproate |
| NSC-17592 |
| 17-alpha-hydroxy-progesterone caproate |
| Corlutin L.A. |
| Gesterol LA 250 |
| 17-OHPC |
| Gestiva |
| Procyte depo |
| Progesterone retard pharlon |
| 17alpha-Caproyloxypregn-4-ene-3,20-dione |
| Delalutin (TN) |
| 17-alpha-Hydroxyprogesterone caproate |
| 17-((1-Oxohexyl)oxy)pregn-4-ene-3,20-dione |
| MLS000028438 |
| 17a-hydroxyprogesterone caproate |
| Oxiprogesterone Caproate |
| Idroprogesterone caproato |
| 17 alpha-hydroxyprogesterone caproate |
| 17alpha-Hydroxyprogesterone hexanoate |
| Hydroxyprogesteroni caproas |
| 17.alpha.-caproyloxy-p4 |
| Progesterone, 17-hydroxy-, hexanoate |
| Caproate d'hydroxyprogesterone |
| SMR000058336 |
| Caproato de hidroxiprogesterona |
| MLS001148643 |
| CHEBI:5812 |
| DTXSID6043915 |
| UNII-276F2O42F5 |
| 17-Hydroxypregn-4-ene-3,20-dione hexanoate |
| 17a-Hydroxyprogesterone hexanoate |
| Idroprogesterone caproato [DCIT] |
| EINECS 211-138-8 |
| Pregn-4-ene-3,20-dione, 17-hydroxy-, hexanoate |
| Pregn-4-ene-3,20-dione, 17-((1-oxohexyl)oxy)- |
| 17-alpha-Hydroxyprogesterone hexanoate |
| 17.alpha.-Hydroxyprogesterone caproate |
| 17alpha-Hydroxyprogesterone n-caproate |
| 276F2O42F5 |
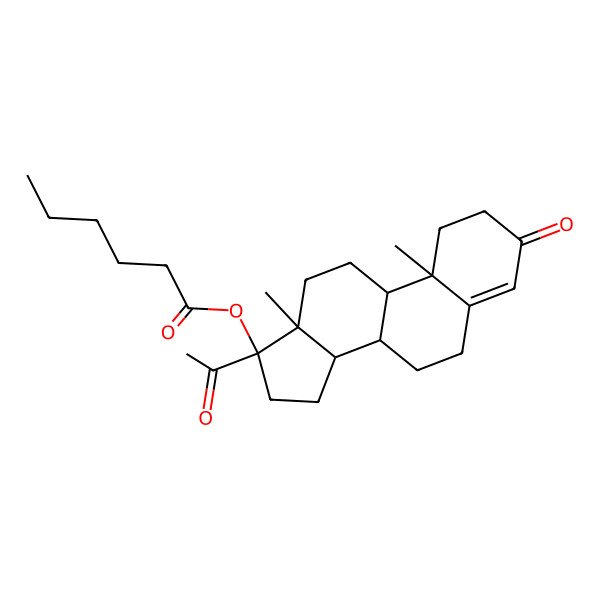
| (8R,9S,10R,13S,14S,17R)-17-acetyl-10,13-dimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl hexanoate |
| 17.alpha.-Hydroxyprogesterone hexanoate |
| Hydroxyprogesteroni caproas [INN-Latin] |
| oxyprogesterone caproate |
| NCGC00021268-03 |
| 17-alpha-Hydroxy progesterone n-caproate |
| 17.alpha.-Hydroxyprogesterone n-caproate |
| 3,20-Dioxo-4-pregnen-17alpha-yl hexanoat |
| 3,20-Dioxopregn-4-en-17alpha-yl caproate |
| Caproate d'hydroxyprogesterone [INN-French] |
| Hydroxyprogesterone caproate [USP:INN:JAN] |
| 17alpha-Hydroxyprogesterone-17alpha caproate |
| 17-alpha-Hexanoyloxypregn-4-ene-3,20-dione |
| Caproato de hidroxiprogesterona [INN-Spanish] |
| 3,20-Dioxopregn-4-en-17.alpha.-yl caproate |
| 17.alpha.-Hydroxypregn-4-ene-3,20-dione hexanoate |
| Pregn-4-ene-3,20-dione, 17-[(1-oxohexyl)oxy]- |
| DTXCID4023915 |
| Hexanoic acid, ester with 17-hydroxypregn-4-ene-3,20-dione |
| CAS-630-56-8 |
| 17|A-Hydroxyprogesterone caproate |
| [(8R,9S,10R,13S,14S,17R)-17-acetyl-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl] hexanoate |
| Lewntogest |
| 17|A-Hydroxyprogesterone hexanoate |
| Hyproval P.A. |
| Hylutin (TN) |
| Makena (TN) |
| Hydroxyprogesterone caproate [USAN:INN:JAN] |
| Proluton depot (TN) |
| Progesterone, hexanoate |
| 17alpha-Hydroxyprogesterone caproate [Progestins] |
| 17-Caproyloxyprogesterone |
| Opera_ID_1479 |
| D00AEQ |
| Deluteval 2X (Salt/Mix) |
| SCHEMBL5330 |
| MLS002207289 |
| 17alpha-Caproyloxyprogesterone |
| 17-hydroxyprogesterone-caproate |
| Caproic acid hydroxyprogesterone |
| cid_169870 |
| CHEMBL1200848 |
| BDBM70293 |
| 17alpha-Hydroxyprogesteron-Caproat |
| HMS2230L08 |
| BCP16081 |
| HY-B0742 |
| NSC17592 |
| Tox21_113502 |
| Tox21_302333 |
| 17-alpha hydroxyprogesterone caproate |
| MFCD00072134 |
| s4674 |
| AKOS005267159 |
| Tox21_113502_1 |
| 17 alpha -Hydroxyprogesterone Caproate |
| CCG-268987 |
| DB06789 |
| GS-3233 |
| NCGC00021268-04 |
| NCGC00255784-01 |
| (1S,11S,15S,2R,10R,14R)-14-acetyl-2,15-dimethyl-5-oxotetracyclo[8.7.0.0<2,7>.0 <11,15>]heptadec-6-en-14-yl hexanoate |
| HYDROXYPROGESTERONE CAPROATE [INN] |
| HYDROXYPROGESTERONE CAPROATE [JAN] |
| Pregn-4-ene-3, 17-hydroxy-, hexanoate |
| 17alpha-hexanoyloxy-4pregnene-3,20-dione |
| Hydroxyprogesterone caproate (JAN/USP/INN) |
| HYDROXYPROGESTERONE CAPROATE [MART.] |
| HYDROXYPROGESTERONE CAPROATE [VANDF] |
| Pregn-4-ene-3, 17-[(1-oxohexyl)oxy]- |
| 17-Hydroxypregn-4-ene-3,20-dione caproate |
| H0994 |
| HYDROXYPROGESTERONE CAPROATE [USP-RS] |
| HYDROXYPROGESTERONE CAPROATE [WHO-DD] |
| 17alpha-Hydroxyprogesterone hexanoate, >=98% |
| C08148 |
| D00949 |
| H10189 |
| 17-.alpha.-Hexanoyloxypregn-4-ene-3,20-dione |
| 17alpha-Hydroxypregn-4-ene-3,20-diene Caproate |
| EN300-7422372 |
| 3,20-Dioxopregn-4-en-17.alpha.-yl hexanoate # |
| A834188 |
| HYDROXYPROGESTERONE CAPROATE [ORANGE BOOK] |
| SR-01000003075 |
| 17.alpha.-Hydroxypregn-4-ene-3,20-dione caproate |
| 17.ALPHA.-HYDROXYPROGESTERONE CAPROATE [MI] |
| HYDROXYPROGESTERONE CAPROATE [USP MONOGRAPH] |
| Q-201222 |
| Q3792032 |
| SR-01000003075-3 |
| 17-Hydroxyprogesterone hexanoate;17-Hydroxyprogesterone caproate |
| Hydroxyprogesterone caproate, United States Pharmacopeia (USP) Reference Standard |
| (1R,3aS,3bR,9aR,9bS,11aS)-1-acetyl-9a,11a-dimethyl-7-oxo-1H,2H,3H,3aH,3bH,4H,5H,7H,8H,9H,9aH,9bH,10H,11H,11aH-cyclopenta[a]phenanthren-1-yl hexanoate |
| (1S,2R,10R,11S,14R,15S)-14-acetyl-2,15-dimethyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-14-yl hexanoate |
| [(10R,13S,17R)-17-acetyl-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl] hexanoate |
| [(8R,9S,10R,13S,14S,17R)-17-acetyl-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl] hexanoate;17-Caproxyprogesterone |
|
There are more than 10 synonyms. If you wish to see them all click here.
|