| neomycin |
| NEOMYCIN B |
| 119-04-0 |
| Fradiomycin |
| Mycifradin |
| Soframycin |
| Fradiomycin B |
| Enterfram |
| Framygen |
| Actilin |
| Neomas |
| Fradiomycinum |
| Framicetina |
| Framycetine |
| Framycetinum |
| Caswell No. 595 |
| Nivemycin |
| Neomycin B sulfate |
| Antibiotic 10676 |
| NEOMYCIN SULFATE |
| 1404-04-2 |
| Neobrettin |
| USAF CB-19 |
| CCRIS 5462 |
| Framidal |
| Framycin |
| Francetin |
| Dekamycin iii |
| HSDB 3242 |
| Soframycine |
| Actiline |
| Framycetin (INN) |
| Neo-Rx |
| Antibiotique |
| Myacyne |
| Neolate |
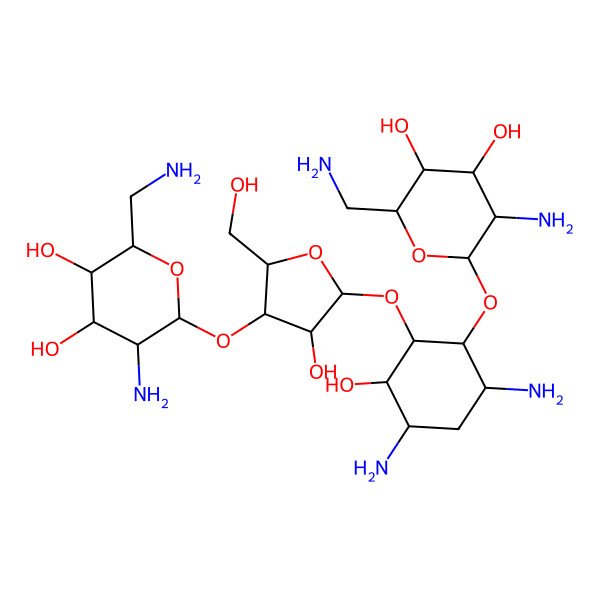
| (2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-[(1R,2R,3S,4R,6S)-4,6-diamino-2-[(2S,3R,4S,5R)-4-[(2R,3R,4R,5S,6S)-3-amino-6-(aminomethyl)-4,5-dihydroxyoxan-2-yl]oxy-3-hydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy-3-hydroxycyclohexyl]oxyoxane-3,4-diol |
| ANTIBIOTIQUE EF 185 |
| Neomycin sulphate |
| CHEBI:7508 |
| Vonamycin powder V |
| Neomcin |
| Neomin |
| 4BOC774388 |
| (1R,2R,3S,4R,6S)-4,6-diamino-2-{[3-O-(2,6-diamino-2,6-dideoxy-beta-L-idopyranosyl)-beta-D-ribofuranosyl]oxy}-3-hydroxycyclohexyl 2,6-diamino-2,6-dideoxy-alpha-D-glucopyranoside |
| Neomicina [DCIT] |
| Framycetinum [INN-Latin] |
| Neomicina |
| Neomycine |
| Neomycinum |
| PIMAVECORT |
| FRAMYCETIN [INN] |
| Neomycine [INN-French] |
| Neomycinum [INN-Latin] |
| Framycetine [INN-French] |
| NMY |
| Framicetina [INN-Spanish] |
| Framycetin [INN:BAN:DCF] |
| Bycomycin |
| Jernadex |
| Fradiomycin B;Neomycin B |
| Neomycin [INN:BAN] |
| EINECS 204-292-2 |
| EINECS 215-766-3 |
| EPA Pesticide Chemical Code 006303 |
| BRN 0101621 |
| Mycerin |
| UNII-I16QD7X297 |
| UNII-4BOC774388 |
| Antibiotic 956 |
| (2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-[(1R,2R,3S,4R,6S)-4,6-diamino-2-[(2S,3R,4S,5R)-4-[(2R,3R,4R,5S,6S)-3-amino-6-(aminomethyl)-4,5-dihydroxy-tetrahydropyran-2-yl]oxy-3-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]oxy-3-hydroxy-cyclohexoxy]tetrahydropyran-3,4-diol |
| NEOMYCINB |
| Antibiotic produced by Streptomyces decaris. Neomycin B |
| NEOMYCIN B [MI] |
| Prestwick3_000158 |
| Neomycin B; Fradiomycin B |
| SCHEMBL3279 |
| FRAMYCETIN [WHO-DD] |
| BSPBio_000296 |
| GTPL709 |
| NEOMYCIN B [USP-RS] |
| 4-18-00-07476 (Beilstein Handbook Reference) |
| D-Streptamine, O-2,6-diamino-2,6-dideoxy-alpha-D-glucopyranosyl-(1-4)-O-(O-2,6-diamino-2,6-dideoxy-beta-L-idopyranosyl-(1-3)-beta-D-ribofuranosyl-(1-5))-2-deoxy- |
| BPBio1_000326 |
| CHEMBL184618 |
| DTXSID2023359 |
| HMS2089P15 |
| I16QD7X297 |
| AKOS024284361 |
| CS-6390 |
| DB00452 |
| NCGC00179612-01 |
| D-Streptamine, O-2,6-diamino-2,6-dideoxy-.beta.-L-idopyranosyl-(1.->3)-O-.beta.-D-ribofuranosyl-(1->5)]-O-[2,6-diamino-2,6-dideoxy-.alpha.-D-glucopyranosyl-(1->4)]-2-deoxy |
| HY-17624 |
| AB00443887 |
| C01737 |
| D05140 |
| AB00443887-03 |
| EN300-7480789 |
| MYCIFRADIN; NEOMAS; PIMAVECORT; VONAMYCIN |
| ANTIBIOTIC PRODUCED BY STREPTOMYCES DECARIS |
| J-004060 |
| Q4492348 |
| BRD-K71013094-065-01-2 |
| (2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-{[(1R,2R,3S,4R,6S)-4,6-diamino-2-{[(2S,3R,4S,5R)-4-{[(2R,3R,4R,5S,6S)-3-amino-6-(aminomethyl)-4,5-dihydroxyoxan-2-yl]oxy}-3-hydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy}-3-hydroxycyclohexyl]oxy}oxane-3,4-diol |
| 11004-65-2 |
| 6-(2-{(4S,2R,3R,5R)-4-[(5S,6S,2R,3R,4R)-3-amino-6-(aminomethyl)-4,5-dihydroxy( 2H-3,4,5,6-tetrahydropyran-2-yloxy)]-3-hydroxy-5-(hydroxymethyl)oxolan-2-yloxy }(3S,6S,1R,2R,4R)-4,6-diamino-3-hydroxycyclohexyloxy)(3S,2R,4R,5R,6R)-5-amino- 2-(aminomethyl)-2H |
| D-STREPTAMINE, O-2,6-DIAMINO-2,6-DIDEOXY-.ALPHA.-D-GLUCOPYRANOSYL-(1->4)-O-(O-2,6-DIAMINO-2,6-DIDEOXY-.BETA.-L-IDOPYRANOSYL-(1->3)-.BETA.-D-RIBOFURANOSYL-(1->5))-2-DEOXY- |
| D-STREPTAMINE, O-2,6-DIAMINO-2,6-DIDEOXY-.BETA.-L-IDOPYRANOSYL-(1->3)-O-.BETA.-D-RIBOFURANOSYL-(1->5)-O-(2,6-DIAMINO-2,6-DIDEOXY-.ALPHA.-D-GLUCOPYRANOSYL-(1->4))-2-DEOXY- |
|
There are more than 10 synonyms. If you wish to see them all click here.
|