Fecosterol
| Internal ID | 2ead864c-01e5-4442-b079-4e2891bd0380 |
| Taxonomy | Lipids and lipid-like molecules > Steroids and steroid derivatives > Ergostane steroids > Ergosterols and derivatives |
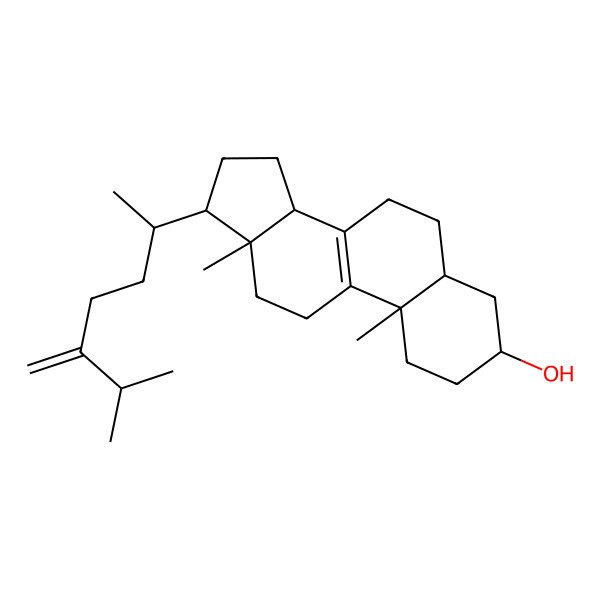
| IUPAC Name | (3S,5S,10S,13R,14R,17R)-10,13-dimethyl-17-[(2R)-6-methyl-5-methylideneheptan-2-yl]-2,3,4,5,6,7,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol |
| SMILES (Canonical) | CC(C)C(=C)CCC(C)C1CCC2C1(CCC3=C2CCC4C3(CCC(C4)O)C)C |
| SMILES (Isomeric) | C[C@H](CCC(=C)C(C)C)[C@H]1CC[C@@H]2[C@@]1(CCC3=C2CC[C@@H]4[C@@]3(CC[C@@H](C4)O)C)C |
| InChI | InChI=1S/C28H46O/c1-18(2)19(3)7-8-20(4)24-11-12-25-23-10-9-21-17-22(29)13-15-27(21,5)26(23)14-16-28(24,25)6/h18,20-22,24-25,29H,3,7-17H2,1-2,4-6H3/t20-,21+,22+,24-,25+,27+,28-/m1/s1 |
| InChI Key | SLQKYSPHBZMASJ-QKPORZECSA-N |
| Popularity | 194 references in papers |
| Molecular Formula | C28H46O |
| Molecular Weight | 398.70 g/mol |
| Exact Mass | 398.354866087 g/mol |
| Topological Polar Surface Area (TPSA) | 20.20 Ų |
| XlogP | 8.10 |
| 516-86-9 |
| 24-Methylene-5alpha-cholest-8-en-3beta-ol |
| delta-8(24),28-Ergostadienol |
| 48A2TY6K38 |
| 24-methylene-cholest-8-en-3beta-ol |
| UNII-48A2TY6K38 |
| (3S,5S,10S,13R,14R,17R)-10,13-dimethyl-17-[(2R)-6-methyl-5-methylideneheptan-2-yl]-2,3,4,5,6,7,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol |
| 8(9), (5-ALPHA)-CHOLESTEN-24-METHYLENE-3-BETA-OL |
| Ergosta-8,24(28)-dien-3-ol, (3beta,5alpha)- |
| (3S,5S,10S,13R,14R,17R)-17-((1R)-1,5-DIMETHYL-4-METHYLENE-HEXYL)-10,13-DIMETHYL-2,3,4,5,6,7,11,12,14,15,16,17-DODECAHYDRO-1H-CYCLOPENTA(A)PHENANTHREN-3-OL |
| There are more than 10 synonyms. If you wish to see them all click here. |
| Target | Value | Probability (raw) | Probability (%) |
|---|---|---|---|
| No predicted properties yet! | |||
Proven Targets:
| CHEMBL ID | UniProt ID | Name | Min activity | Assay type | Source |
|---|---|---|---|---|---|
| No proven targets yet! | |||||
Predicted Targets (via Super-PRED):
| CHEMBL ID | UniProt ID | Name | Probability | Model accuracy |
|---|---|---|---|---|
| CHEMBL253 | P34972 | Cannabinoid CB2 receptor | 98.44% | 97.25% |
| CHEMBL5619 | P27695 | DNA-(apurinic or apyrimidinic site) lyase | 96.77% | 91.11% |
| CHEMBL3251 | P19838 | Nuclear factor NF-kappa-B p105 subunit | 95.57% | 96.09% |
| CHEMBL221 | P23219 | Cyclooxygenase-1 | 94.63% | 90.17% |
| CHEMBL3137262 | O60341 | LSD1/CoREST complex | 94.12% | 97.09% |
| CHEMBL4203 | Q9HAZ1 | Dual specificity protein kinase CLK4 | 90.76% | 94.45% |
| CHEMBL1871 | P10275 | Androgen Receptor | 88.24% | 96.43% |
| CHEMBL2581 | P07339 | Cathepsin D | 87.41% | 98.95% |
| CHEMBL1994 | P08235 | Mineralocorticoid receptor | 87.09% | 100.00% |
| CHEMBL1907603 | Q05586 | Glutamate NMDA receptor; GRIN1/GRIN2B | 86.66% | 95.89% |
| CHEMBL4026 | P40763 | Signal transducer and activator of transcription 3 | 86.37% | 82.69% |
| CHEMBL4681 | P42330 | Aldo-keto-reductase family 1 member C3 | 86.28% | 89.05% |
| CHEMBL5608 | Q16288 | NT-3 growth factor receptor | 85.42% | 95.89% |
| CHEMBL2373 | P21730 | C5a anaphylatoxin chemotactic receptor | 85.19% | 92.62% |
| CHEMBL5845 | P23415 | Glycine receptor subunit alpha-1 | 83.43% | 90.71% |
| CHEMBL238 | Q01959 | Dopamine transporter | 82.38% | 95.88% |
| CHEMBL3130 | O00329 | PI3-kinase p110-delta subunit | 82.24% | 96.47% |
| CHEMBL4394 | Q9NYA1 | Sphingosine kinase 1 | 81.21% | 96.03% |
| CHEMBL1795139 | Q8IU80 | Transmembrane protease serine 6 | 81.05% | 98.33% |
| CHEMBL3807 | P17706 | T-cell protein-tyrosine phosphatase | 80.99% | 93.00% |
| CHEMBL237 | P41145 | Kappa opioid receptor | 80.13% | 98.10% |
| CHEMBL1907605 | P24864 | Cyclin-dependent kinase 2/cyclin E1 | 80.03% | 92.88% |
| PubChem | 440371 |
| LOTUS | LTS0185843 |
| wikiData | Q15410975 |