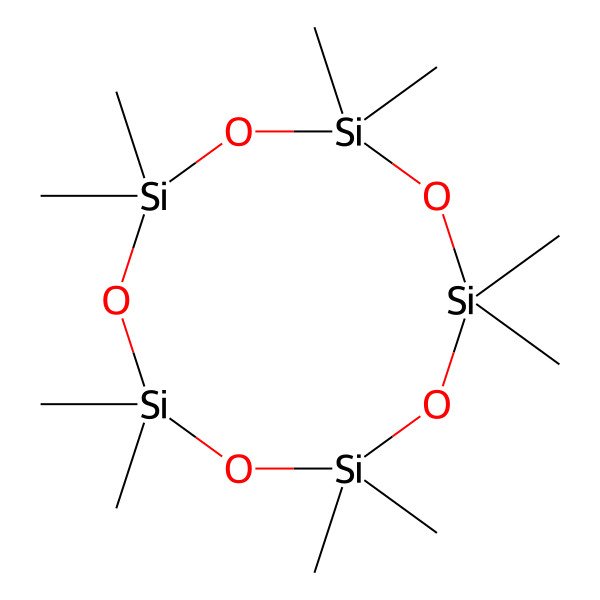
| 541-02-6 |
| Cyclopentasiloxane, decamethyl- |
| Cyclomethicone 5 |
| 2,2,4,4,6,6,8,8,10,10-Decamethyl-1,3,5,7,9,2,4,6,8,10-pentaoxapentasilecane |
| Dimethylsiloxane pentamer |
| Dekamethylcyklopentasiloxan |
| NUC silicone VS 7158 |
| Dow corning 345 |
| Silicon SF 1202 |
| Cyclic dimethylsiloxane pentamer |
| Ciclopentasiloxane |
| Cyclomethicone D5 |
| KF 995 |
| VS 7158 |
| CCRIS 1328 |
| HSDB 5683 |
| Dekamethylcyklopentasiloxan [Czech] |
| EINECS 208-764-9 |
| UNII-0THT5PCI0R |
| 0THT5PCI0R |
| SF 1202 |
| BRN 1800166 |
| C10H30O5Si5 |
| DTXSID1027184 |
| D5 |
| EC 208-764-9 |
| 4-04-00-04128 (Beilstein Handbook Reference) |
| Cyclopentasiloxane, 2,2,4,4,6,6,8,8,10,10-decamethyl- |
| MFCD00046966 |
| 2,2,4,4,6,6,8,8,10,10-decamethyl-1,3,5,7,9,2,4,6,8,10-pentoxapentasilecane |
| D5-sil |
| Ddecamethylcyclopentasiloxane |
| decamethyl cyclopentasiloxane |
| D5 Cyclomethicone |
| dimethylcyclopentasiloxane |
| Decamethylcylopentasiloxane |
| JEESILC CPS-211 |
| SCHEMBL28497 |
| N-Propylheptamethyltrisiloxane |
| XIAMETER PMX-0245 |
| DTXCID907184 |
| CYCLOPENTASILOXANE (D5) |
| 2,2,4,4,6,6,8,8,10,10-Decamethylcyclopentasiloxane |
| CHEMBL1885178 |
| CYCLOPENTASILOXANE [INCI] |
| D5 (Decamethylcyclopentasiloxane) |
| CHEBI:191092 |
| Decamethylcyclopentasiloxane, 97% |
| XMSXQFUHVRWGNA-UHFFFAOYSA-N |
| C10-H30-O5-Si5 |
| CYCLOMETHICONE 5 [USP-RS] |
| CYCLOMETHICONE 5 [WHO-DD] |
| BCP15826 |
| Tox21_303170 |
| CD3770 |
| KF-995 |
| AKOS008901199 |
| CS-O-01236 |
| CS-W009767 |
| DB11244 |
| DOW CORNING ST CYCLOMETHICONE 5 |
| DECAMETHYLCYCLOPENTASILOXANE [MI] |
| NCGC00163981-01 |
| NCGC00257224-01 |
| OCTAMETHYLCYCLOTETRASILOXANE (D5) |
| AS-59731 |
| CAS-541-02-6 |
| DECAMETHYLCYCLOPENTASILOXANE [HSDB] |
| LS-58254 |
| KP-545 COMPONENT CYCLOMETHICONE 5 |
| D1890 |
| D3770 |
| Decamethylcyclopentasiloxane (cyclic monomer) |
| FT-0665531 |
| D78203 |
| S05475 |
| Decamethylcyclopentasiloxane, analytical standard |
| Q414350 |
| Ciclopentasiloxano, 2,2,4,4,6,6,8,8,10,10-decametil- |
| decamethyl-1,3,5,7,9,2,4,6,8,10-pentaoxapentasilecane |
| Cyclomethicone 5, United States Pharmacopeia (USP) Reference Standard |
| 2,2,4,4,6,6,8,8,10,10-Decamethyl-1,3,5,7,9,2,4,6,8,10-pentaoxapentasilecane # |
| D5 Cyclomethicone, Pharmaceutical Secondary Standard; Certified Reference Material |
|
There are more than 10 synonyms. If you wish to see them all click here.
|