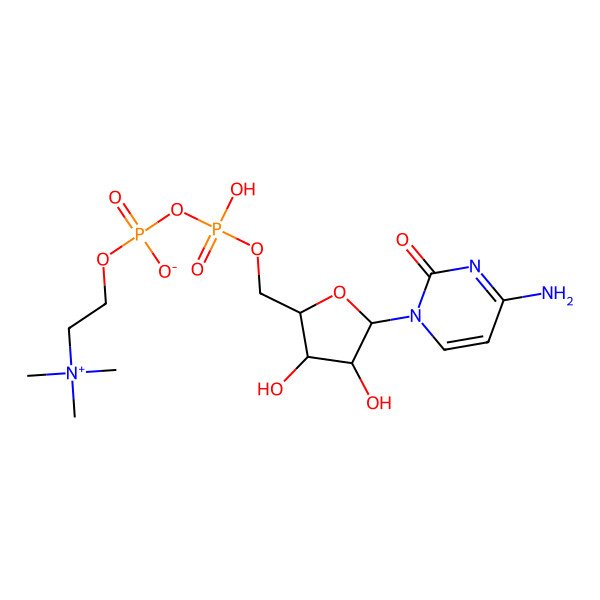
| cytidine 5'-diphosphocholine |
| CDP-choline |
| 987-78-0 |
| citicholine |
| cytidine diphosphate choline |
| Citidoline |
| Cytidoline |
| Difosfocin |
| Cyscholin |
| Citicolinum |
| Citicolina |
| CDP-colina |
| cyticholine |
| Cytidine 5'-diphosphoric choline |
| Haocolin |
| Nicholin |
| Niticolin |
| Recofnan |
| Recognan |
| Somazina |
| Suncholin |
| Colite |
| Ensign |
| Cytidindiphosphocholin |
| Cytidine 5'-(choline diphosphate) |
| Cytidine 5'-(cholinyl pyrophosphate) |
| Cytidine diphosphocholine |
| Cytidine diphosphorylcholine |
| Choline cytidine diphosphate |
| Cytidine choline diphosphate |
| citidin difosfato de colina |
| Emicholin |
| Meibis |
| Choline 5'-cytidine diphosphate |
| CDP-cholin |
| Cereb |
| Cytidine 5'-diphosphate choline |
| Cytidine diphosphate choline ester |
| Cytidinediphosphoric choline |
| [2-CYTIDYLATE-O'-PHOSPHONYLOXYL]-ETHYL-TRIMETHYL-AMMONIUM |
| Cytidine 5'-(trihydrogen diphosphate), P'-(2-(trimethylammonio)ethyl) ester, inner salt |
| IP 302 |
| IP-302 |
| Choline cytidine 5'-pyrophosphate ester |
| Choline, ester with cytidine 5'-pyrophosphate |
| CDP-cholin [German] |
| CDP-colina [Spanish] |
| Startonyl |
| Ubelin |
| Citicoline [INN:JAN] |
| Citicolinum [INN-Latin] |
| Citicolina [INN-Spanish] |
| 536BQ2JVC7 |
| CHEBI:16436 |
| {2-[({[(2R,3S,4R,5R)-5-(4-amino-2-oxo-1,2-dihydropyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl phosphonato)oxy]ethyl}trimethylazanium |
| 2-(((((((2R,3S,4R,5R)-5-(4-Amino-2-oxopyrimidin-1(2H)-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(hydroxy)phosphoryl)oxy)oxidophosphoryl)oxy)-N,N,N-trimethylethanaminium |
| Cytidindiphosphocholin [German] |
| Cytidine diphosphate cholin ester |
| EINECS 213-580-7 |
| Citidin difosfato de colina [Spanish] |
| Cytidine 5-diphosphocholine |
| NSC 122002 |
| BRN 4170138 |
| UNII-536BQ2JVC7 |
| Cytidine, 5'-pyrophosphate, ester with choline |
| Cytidine 5'-(trihydrogen diphosphate), P'-[2-(trimethylammonio)ethyl] ester, inner salt |
| Nicholin (TN) |
| Citicoline (JP17/INN) |
| cytidine diphosphate-choline |
| CDP-choline (neutral charge) |
| SCHEMBL221081 |
| DTXSID9048431 |
| RZZPDXZPRHQOCG-OJAKKHQRSA-N |
| MFCD00868097 |
| AKOS025312525 |
| CCG-269597 |
| DB04290 |
| DB12153 |
| Choline, hydroxide, 5'-ester with cytidine 5'-(trihydrogen pyrophosphate), inner salt |
| Cytidine 5'-(trihydrogen diphosphate), mono(2-(trimethylammonio)ethyl) ester, hydroxide, inner salt |
| C3438 |
| C00307 |
| D00057 |
| D81851 |
| EN300-7398882 |
| A847982 |
| W-100063 |
| Q28529682 |
| Cytidine diphosphate-choline;CDP-Choline;Cytidine 5'-diphosphocholine |
| 5'-O-[(S)-hydroxy({[2-(trimethylammonio)ethoxy]phosphinato}oxy)phosphoryl]cytidine |
| 5'-O-[hydroxy({[2-(trimethylammonio)ethoxy]phosphinato}oxy)phosphoryl]cytidine |
| Cytidine 5'-(trihydrogen diphosphate), P'-[2-(trimethylammonio)ethyl]ester, inner salt |
|
There are more than 10 synonyms. If you wish to see them all click here.
|