| 60925-61-3 |
| Precef |
| Ceforanido |
| Ceforanidum |
| Ceforanidum [INN-Latin] |
| Ceforanido [INN-Spanish] |
| BL-S786 |
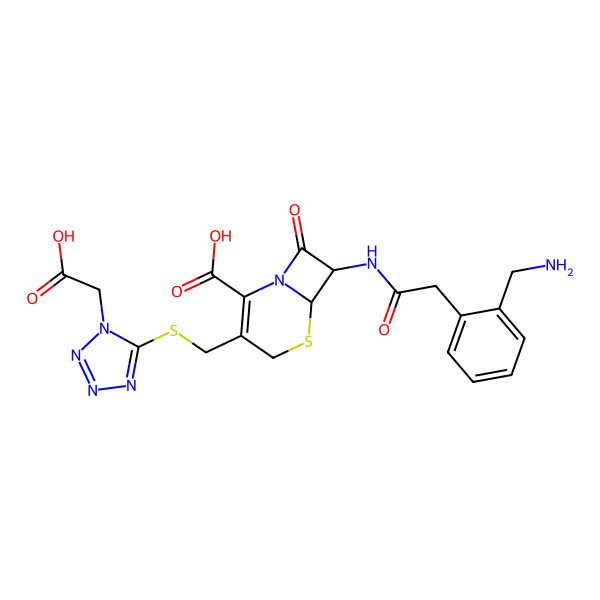
| (6R,7R)-7-[[2-[2-(aminomethyl)phenyl]acetyl]amino]-3-[[1-(carboxymethyl)tetrazol-5-yl]sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| UNII-8M1YF8951V |
| CHEBI:3495 |
| DTXSID1022760 |
| 8M1YF8951V |
| (6R,7R)-7-{2-[2-(aminomethyl)phenyl]acetamido}-3-({[1-(carboxymethyl)-1H-1,2,3,4-tetrazol-5-yl]sulfanyl}methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| 7-(o-(Aminomethyl)phenylacetamido)-3-(((1-(carboxymethyl)-1H-tetrazol-5-yl)thio)methyl)-3-cephem-4-carboxylic acid |
| NSC-760049 |
| DTXCID402760 |
| (6R,7R)-7-(2-(alpha-Amino-o-tolyl)acetamido)-3-(((1-(carboxymethyl)-1H-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid |
| Ceforanide [USAN:USP:INN:BAN] |
| NSC 760049 |
| (6R-trans)-7-(((2-(Aminomethyl)phenyl)acetyl)amino)-3-(((1-(carboxymethyl)-1H-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid |
| 5-Thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 7-(((2-(aminomethyl)phenyl)acetyl)amino)-3-(((1-(carboxymethyl)-1H-tetrazol-5-yl)thio)methyl)-8-oxo-, (6R-trans)- |
| Ceforanidum (INN-Latin) |
| Ceforanido (INN-Spanish) |
| CEFORANIDE (MART.) |
| CEFORANIDE [MART.] |
| CEFORANIDE (USP-RS) |
| CEFORANIDE [USP-RS] |
| 7-[O-(aminomethyl)phenylacetamido]-3-[[[1-(carboxymethyl)-1H-tetrazol-5-yl]thio]methyl]-3-cephem-4-carboxylic acid |
| 7beta-[2-(aminomethyl)phenyl]acetamido-3-{[1-(carboxymethyl)-1H-tetrazol-5-yl]sulfanyl}methyl-3,4-didehydrocepham-4-carboxylic acid |
| CEFORANIDE (USP IMPURITY) |
| CEFORANIDE [USP IMPURITY] |
| Ceforanide (USAN:USP:INN:BAN) |
| BL-S 786 |
| (6R,7R)-7-({[2-(aminomethyl)phenyl]acetyl}amino)-3-({[1-(carboxymethyl)-1H-tetrazol-5-yl]sulfanyl}methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| Precef (TN) |
| Ceforanide (USP/INN) |
| SR-01000872614 |
| cefaronide |
| ceforanida |
| NCGC00016897-01 |
| (6R,7R)-7-(((2-(aminomethyl)phenyl)acetyl)amino)-3-(((1-(carboxymethyl)-1H-tetrazol-5-yl)sulfanyl)methyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid |
| (6R,7R)-7-((2-(2-(aminomethyl)phenyl)acetyl)amino)-3-((1-(carboxymethyl)tetrazol-5-yl)sulfanylmethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid |
| (6R,7R)-7-[2-(alpha-Amino-O-tolyl)acetamido]-3-[[[1-(carboxymethyl)-1H-tetrazol-5-yl]thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| 7beta-(2-(aminomethyl)phenyl)acetamido-3-((1-(carboxymethyl)-1H-tetrazol-5-yl)sulfanyl)methyl-3,4-didehydrocepham-4-carboxylic acid |
| CAS-60925-61-3 |
| CEFORANIDE [MI] |
| CEFORANIDE [INN] |
| Prestwick0_000470 |
| Prestwick1_000470 |
| Prestwick2_000470 |
| Prestwick3_000470 |
| CEFORANIDE [USAN] |
| CEFORANIDE [VANDF] |
| CEFORANIDE(200MG) |
| CEFORANIDE [WHO-DD] |
| BSPBio_000580 |
| SCHEMBL122072 |
| SPBio_002519 |
| BPBio1_000638 |
| CEFORANIDE [ORANGE BOOK] |
| CHEMBL1201046 |
| GTPL12218 |
| J01DC11 |
| HMS1569M22 |
| HMS2096M22 |
| HMS3713M22 |
| (6R,7R)-7-(2-(2-(Aminomethyl)phenyl)acetamido)-3-(((1-(carboxymethyl)-1H-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| HY-B1297 |
| Tox21_110670 |
| AKOS027427040 |
| CCG-220470 |
| DB00923 |
| NCGC00179514-05 |
| (6R,7R)-7-({[2-(aminomethyl)phenyl]acetyl}amino)-3-({[1-(carboxymethyl)-1H-tetrazol-5-yl]thio}methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| AS-15783 |
| AB00513845 |
| CS-0013066 |
| S5081 |
| C06884 |
| D00259 |
| D81832 |
| EN300-7408761 |
| A913663 |
| Q5057287 |
| SR-01000872614-2 |
| SR-01000872614-3 |
| BRD-K37848908-001-03-1 |
| (6R,7R)-7-(2-(2-(Aminomethyl)phenyl)acetamido)-3-(((1-(carboxymethyl)-1H-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylicacid |
| 7-(alpha-(2-aminomethylphenyl)acetamido)-3-((1-carboxymethyltetrazol-5-ylthio)methyl)-3-cephem-4-carboxylic acid |
| 7-[[2-[2-(aminomethyl)phenyl]acetyl]amino]-3-[[1-(carboxymethyl)tetrazol-5-yl]sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
|
There are more than 10 synonyms. If you wish to see them all click here.
|