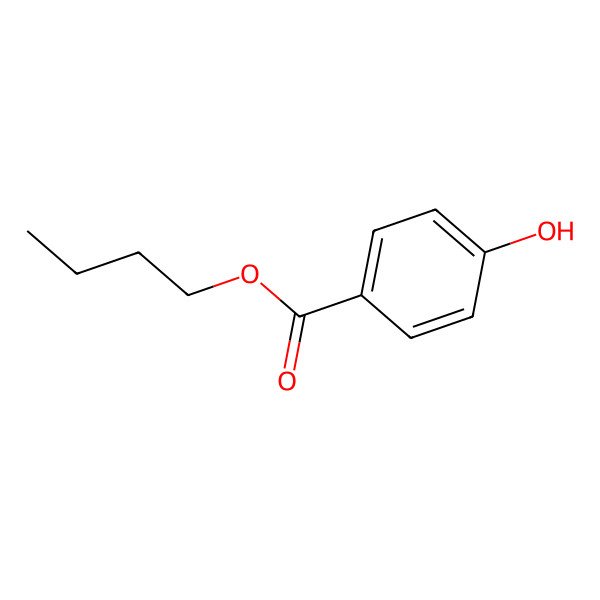
| Butyl 4-hydroxybenzoate |
| 94-26-8 |
| Butyl paraben |
| Butyl p-hydroxybenzoate |
| Butyl parahydroxybenzoate |
| Nipabutyl |
| Butoben |
| Butyl chemosept |
| Butyl parasept |
| Tegosept B |
| Butyl tegosept |
| Butyl butex |
| Tegosept Butyl |
| Aseptoform butyl |
| Preserval B |
| Solbrol B |
| n-Butyl p-hydroxybenzoate |
| Butyl-Parasept |
| Benzoic acid, 4-hydroxy-, butyl ester |
| 4-(Butoxycarbonyl)phenol |
| butyl p-hydroxy benzoate |
| n-butyl 4-hydroxybenzoate |
| n-Butyl parahydroxybenzoate |
| n-Butyl hydroxybenzoate |
| FEMA No. 2203 |
| n-Butyl-p-hydroxybenzoate |
| 4-Hydroxybenzoic acid butyl ester |
| Benzoic acid, p-hydroxy-, butyl ester |
| n-Butyl-paraben |
| p-Hydroxybenzoic acid butyl ester |
| Lexgard B |
| n-Butyl-4-hydroxybenzoate |
| NSC 8475 |
| p-Hydroxybenzoic acid n-butyl ester |
| Butylparaben (NF) |
| Butylparaben (TN) |
| Butylparaben [NF] |
| SPF |
| p-Hydroxybenzoic acid, butyl ester |
| 4-Hydroxybenzoic acid, butyl ester |
| MFCD00016478 |
| p-Hydroxybenzoic butyl ester |
| 3QPI1U3FV8 |
| Butyl parahydroxybenzoate (TN) |
| DTXSID3020209 |
| CHEBI:88542 |
| 4-Hydroxybenzoic acid-n-butyl ester |
| NSC-13164 |
| CAS-94-26-8 |
| NCGC00016354-03 |
| DTXCID90209 |
| Butylparaben [USAN] |
| butyl-p-hydroxybenzoate |
| Caswell No. 130A |
| FEMA Number 2203 |
| n-Butyl paraben |
| 4-Hydroxybenzoic acid-n-butyl ester 1000 microg/mL in Acetonitrile |
| Butyl Par asept |
| SMR000462402 |
| CCRIS 2462 |
| HSDB 286 |
| p-Hydroxy butyl benzoate |
| SR-01000389296 |
| EINECS 202-318-7 |
| UNII-3QPI1U3FV8 |
| EPA Pesticide Chemical Code 061205 |
| BRN 1103741 |
| butyl-paraben |
| AI3-02930 |
| 27K |
| 4mg9 |
| Prestwick0_000894 |
| Prestwick1_000894 |
| Prestwick2_000894 |
| Prestwick3_000894 |
| BUTYLPARABEN [II] |
| BUTYLPARABEN [MI] |
| WLN: QR DVO4 |
| BUTYLPARABEN [HSDB] |
| BUTYLPARABEN [INCI] |
| cid_7184 |
| SCHEMBL3647 |
| BUTYLPARABEN [VANDF] |
| BSPBio_000708 |
| Butyl //p//-Hydroxybenzoate |
| MLS000575004 |
| MLS002154054 |
| MLS002303045 |
| BIDD:ER0231 |
| BUTYLPARABEN [USP-RS] |
| SPBio_002917 |
| BPBio1_000780 |
| CHEMBL459008 |
| F0266-0124 |
| N-Butyl-P-Hydroxybenzoate,(S) |
| BDBM23448 |
| FEMA 2203 |
| NSC8475 |
| Butyl parahydroxybenzoate (JP15) |
| Butyl parahydroxybenzoate (JP17) |
| Butyl 4-hydroxybenzoate, >=99% |
| HMS1570D10 |
| HMS2094A21 |
| HMS2097D10 |
| HMS2220G15 |
| HMS3327P04 |
| HMS3714D10 |
| Pharmakon1600-01505995 |
| HY-B1431 |
| NSC-8475 |
| Tox21_110393 |
| Tox21_201785 |
| Tox21_300332 |
| NSC759303 |
| p-Hydroxybenzoic acid, n-butyl ester |
| s4584 |
| BUTYL HYDROXYBENZOATE [MART.] |
| AKOS000121421 |
| BUTYL HYDROXYBENZOATE [WHO-DD] |
| Tox21_110393_1 |
| BUTYL PARAHYDROXYBENZOATE [JAN] |
| CCG-213596 |
| CS-4783 |
| DB14084 |
| NSC-759303 |
| BUTYL P-HYDROXY BENZOATE [FHFI] |
| Butyl 4-Hydroxybenzoate (Butyl Paraben) |
| NCGC00016354-01 |
| NCGC00016354-02 |
| NCGC00016354-04 |
| NCGC00016354-05 |
| NCGC00016354-06 |
| NCGC00016354-07 |
| NCGC00016354-11 |
| NCGC00091142-01 |
| NCGC00091142-02 |
| NCGC00254294-01 |
| NCGC00259334-01 |
| AC-34535 |
| AS-14309 |
| SY032891 |
| SBI-0206946.P001 |
| Butyl 4-hydroxybenzoate, >=99.0% (GC) |
| AB00513951 |
| B3771 |
| FT-0623315 |
| H0210 |
| EN300-21566 |
| A16382 |
| BUTYL PARAHYDROXYBENZOATE [EP IMPURITY] |
| BUTYL PARAHYDROXYBENZOATE [EP MONOGRAPH] |
| D01420 |
| AB00513951_09 |
| A844895 |
| Q3302873 |
| SR-01000389296-1 |
| SR-01000389296-3 |
| W-100204 |
| BRD-K08287586-001-03-6 |
| BRD-K08287586-001-08-5 |
| Butyl 4-hydroxybenzoate, SAJ first grade, >=99.0% |
| PROPYL HYDROXYBENZOATE IMPURITY D [EP IMPURITY] |
| Z291799028 |
| METHYL PARAHYDROXYBENZOATE IMPURITY D [EP IMPURITY] |
| InChI=1/C11H14O3/c1-2-3-8-14-11(13)9-4-6-10(12)7-5-9/h4-7,12H,2-3,8H2,1H |
|
There are more than 10 synonyms. If you wish to see them all click here.
|