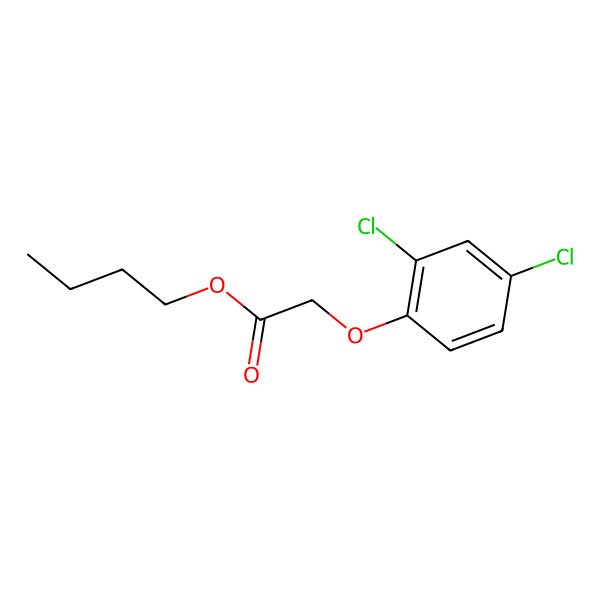
| Butyl 2,4-dichlorophenoxyacetate |
| butyl 2-(2,4-dichlorophenoxy)acetate |
| 2,4-D BUTYL ESTER |
| 2,4-D-butyl |
| Lironox |
| 2,4-D n-butyl ester |
| Butyl ester 2,4-D |
| Butapon |
| Esso Herbicide 10 |
| 2,4-D 1-butyl ester |
| Butyl (2,4-dichlorophenoxy)acetate |
| Shell 40 |
| Butyl 2,4-D |
| Acetic acid, (2,4-dichlorophenoxy)-, butyl ester |
| Butyl ester of 2,4-D |
| Butyl dichlorophenoxyacetate |
| Component Orange |
| Purple Component |
| Hi-Ester 2,4-D |
| The Crop Rider |
| Line Rider 4T |
| 2,4-D-butyl [ISO] |
| Orange II Component |
| Lironox 326 |
| Weed-Rhap B-4D |
| Weed-Rhap B-6D |
| Butyl(2,4-dichlorophenoxy) acetate |
| 2,4-Dichlorophenoxyacetic acid butyl ester |
| Miller's 4# Ester |
| Miller's 6# Ester |
| 2,4-Dichlorophenoxyacetic acid n-butyl ester |
| Butyl 400 |
| Crop Rider 2.67D |
| Caswell No. 315AL |
| Felco HV2 Weed Killer |
| Felco HV4 Weed Killer |
| 2,4-DBE |
| T-H Ded-Weed ME-6 |
| T-H Ded-Weed ME-9 |
| Weed-Rhap B-2.67D |
| Green Cross Weed-No-More |
| MFA No. 4 Weed Killer |
| MFA No. 6 Weed Killer |
| Diamond Shamrock Butyl 4D |
| De-Pester Ded-Weed ME-4 |
| De-Pester Ded-Weed ME-5 |
| De-Pester Ded-Weed ME-6 |
| De-Pester Ded-Weed ME-9 |
| FS Ester 400 Weed Killer |
| 2,4-d butylate |
| Monsanto 2,4-D Butyl Ester |
| NSC 409767 |
| HSDB 1633 |
| Rhodia 2,4-D Butyl Ester 6L |
| Chipman 2,4-D Butyl Ester 4E |
| Chipman 2,4-D Butyl Ester 6E |
| Unico 2,4-D Ester Weed Killer |
| Olin Butyl Ester D4 Weed Killer |
| Olin Butyl Ester D6 Weed Killer |
| MFA 40% Butyl Ester Weed Killer |
| Barber's 2,4-D Ester Weed Killer |
| Chipman 2,4-D Butyl Ester 334E |
| EINECS 202-364-8 |
| Olin Butyl Ester D267 Weed Killer |
| Abco W.K-67 2,4-D Weed Killer |
| Amoco 2,4-D Weed Killer No. 6B |
| General Chemical 2,4-D Butyl Ester Weed Killer |
| UNII-6GO3LVR34R |
| Barco Weed Killer (ester Formulation) |
| Diamond Shamrock Butyl 6D Weed Killer |
| EPA Pesticide Chemical Code 030056 |
| Techne Butyl Ester Weed Killer No. 4 |
| Techne Butyl Ester Weed Killer No. 6 |
| BRN 2056085 |
| 6GO3LVR34R |
| Parsons 2,4-D Weed Killer Butyl Ester |
| Techne 40% Butyl Ester Type Weed Killer |
| AI3-08686 |
| MLS002152860 |
| Sure Death No. 4 Butyl Ester Weed Killer |
| Sure Death No. 6 Butyl Ester Weed Killer |
| Felco Butyl Ester 600 2,4-D Weed Killer |
| DTXSID5020443 |
| Sure Death 40% Butyl Ester Type Weedkiller |
| n-Butylester kyseliny 2,4-dichlorfenoxyoctove |
| (2,4-Dichlorophenoxy)acetic acid, butyl ester |
| 2,4-Dichlorophenoxyacetic acid, n-butyl ester |
| Floratox 428 4-Pound 2,4-D Ester Weed Killer |
| Associated Sales 4-Pound 2,4-D Ester Weed Killer |
| General Chemical 2,4-D 4-Butyl Ester Weed Killer |
| Patterson's Hi-Test Butyl Ester 2,4-D Weed Killer |
| General Chemical 2,4-D 3.34 Butyl Ester Weed Killer |
| General Chemical 2,4-D 6.00 Butyl Ester Weed Killer |
| n-Butylester kyseliny 2,4-dichlorfenoxyoctove [Czech] |
| NSC-409767 |
| butyl [(2,4-dichlorophenyl)oxy]acetate |
| SMR001224484 |
| DTXCID10443 |
| CAS-94-80-4 |
| 2,4-D Butyl Ester ((2,4-Dichlorophenoxy)acetic Acid Butyl Ester) |
| Butyl 2,4-dichlorophenoxyacetate |
| (2, butyl ester |
| 2,4-d, butyl ester |
| 2,4-d, n-butyl ester |
| 2,4-D-1-butyl ester |
| cid_7206 |
| WLN: GR CG DO1VO4 |
| SCHEMBL1226040 |
| CHEMBL1446511 |
| BDBM74250 |
| butyl 2,4-dichloro-phenoxyacetate |
| UQMRAFJOBWOFNS-UHFFFAOYSA-N |
| 2,4-D BUTYL ESTER [MI] |
| HY-B0867 |
| Tox21_201237 |
| Tox21_302757 |
| 2,4-Dichlorophenoxyacetic acid butyl |
| butyl2-(2,4-dichlorophenoxy)acetate |
| MFCD00126845 |
| NSC409767 |
| STL301520 |
| 2,4-dichlorophenoxyacetic butyl ester |
| AKOS005943535 |
| LS-1968 |
| Butyl 2-(2,4-dichlorophenoxy)ethanoate |
| NCGC00090826-01 |
| NCGC00090826-02 |
| NCGC00090826-03 |
| NCGC00256335-01 |
| NCGC00258789-01 |
| AC-12695 |
| AS-76740 |
| CS-0012882 |
| FT-0710757 |
| (2,4-Dichlorophenoxy)acetic acid butyl ester |
| Acetic acid,4-dichlorophenoxy)-, butyl ester |
| butyl 2-[2,4-bis(chloranyl)phenoxy]ethanoate |
| EN300-112152 |
| 2-(2,4-dichlorophenoxy)acetic acid butyl ester |
| 2,4-D-1-butyl ester 100 microg/mL in Cyclohexane |
| 2,4-DICHLOROPHENOXYACETIC ACID, BUTYL ESTER |
| W-100183 |
| Q27264880 |
| Z54191175 |
| 2,4-D 1-butyl ester, PESTANAL(R), analytical standard |
|
There are more than 10 synonyms. If you wish to see them all click here.
|