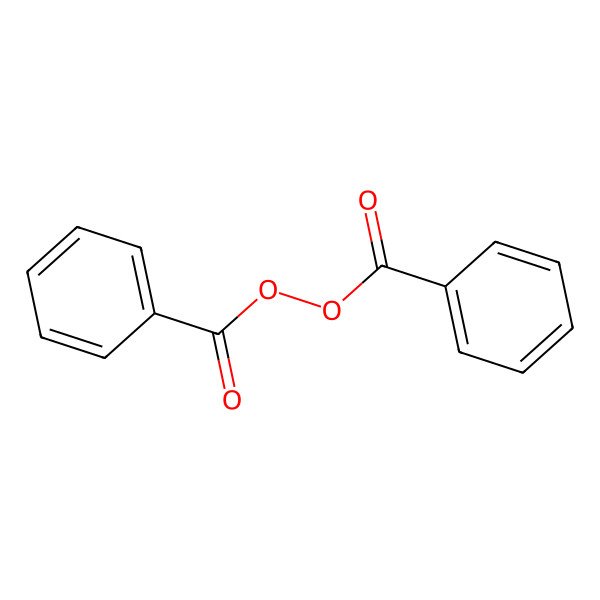
| 94-36-0 |
| Peroxide, dibenzoyl |
| Dibenzoyl peroxide |
| Benzoyl superoxide |
| Benzoperoxide |
| Acetoxyl |
| Benoxyl |
| Lucidol |
| Panoxyl |
| Dibenzoylperoxid |
| Benzol peroxide |
| Benzoylperoxid |
| Asidopan |
| Mytolac |
| Oxylite |
| Persadox |
| Eloxyl |
| Resdan Akne |
| Diphenylglyoxal peroxide |
| Epi-Clear |
| Akneroxid 5 |
| Dry and Clear |
| Duresthin 5 |
| Benzoylperoxyde |
| Luperco AST |
| Loroxide |
| Nayper BO |
| Theraderm |
| Benzac |
| benzoyl benzenecarboperoxoate |
| Dibenzoylperoxyde |
| Peroxyde de benzoyle |
| Persa-Gel |
| Lucidol B 50 |
| Lucidol G 20 |
| benzoylperoxide |
| Perossido di benzoile |
| Benzaknen |
| Debroxide |
| Desanden |
| Novadelox |
| Vanoxide |
| Acnegel |
| Incidol |
| Garox |
| Topex |
| Xerac |
| Benzoic acid, peroxide |
| Akneroxide L |
| Luperox fl |
| Cadox bs |
| Quinolor compound |
| Desquam E |
| Luperco AA |
| Cadox B |
| Nayper B and bo |
| Aztec BPO |
| Benzashave |
| Benzagel |
| Benzagel 10 |
| Brevoxyl |
| Lucidol (peroxide) |
| Norox bzp-250 |
| Norox bzp-C-35 |
| Cadox 40E |
| Desquam X |
| Desquam-X |
| Benbel C |
| Benzac W |
| Clear By Design |
| Cadat BPO |
| Aksil 5 |
| Lucidol 50P |
| Lucidol-70 |
| Lucidol KL 50 |
| Oxy-10 Cover |
| Superox 744 |
| Chaloxyd BP 50FT |
| Abcat 40 |
| Benox 50 |
| Acne-Aid Cream |
| Cadox B 40E |
| Cadox B 50P |
| Cadox B 70W |
| Oxy-5 |
| Cadox B-CH 50 |
| Benprox |
| Benzaknew |
| Benzefoam |
| Lavoclen |
| Stri-dex B.P. |
| Benoxyl (5&10) Lotion |
| Pacnex |
| Cadet |
| Diphenylperoxyanhydride |
| benzoic peroxyanhydride |
| Epi Clear Antiseptic Lotion |
| Neobenz micro |
| Oxy 5 |
| BZF-60 |
| Fostex BPO |
| OXY-10 |
| pHisoAc BP |
| Peroxide, Benzoyl |
| Xerac BP |
| Benzoyl peroxide [USAN] |
| Lucidol 75FP |
| Superoxide, Benzoyl |
| Benzoyl peroxide gel |
| Clearasil Antibacterial Acne Lotion |
| EPSOLAY |
| CCRIS 630 |
| OXY WASH |
| Peroxyderm |
| Benzoyl |
| Dermoxyl |
| HSDB 372 |
| Luzidol |
| Nericur |
| Peroxydex |
| Preoxydex |
| Sanoxit |
| Superox |
| Benzoylperoxid [German] |
| Benzoylperoxyde [Dutch] |
| Bepio |
| benzoyl-peroxide |
| dibenzoylperoxide |
| Benzoyl peroxyde |
| Ins no.928 |
| Diphenylglyoxal Superoxide |
| NSC 671 |
| NSC-671 |
| NSC-675 |
| Akneroxid L |
| Anhydrous benzoyl peroxide |
| Benzoyl peroxide anhydrous |
| Dibenzoylperoxid [German] |
| Dibenzoylperoxyde [Dutch] |
| UNII-W9WZN9A0GM |
| B 75W |
| Clearasil BP Acne Treatment Cream |
| W9WZN9A0GM |
| Superoxide, Diphenylglyoxal |
| Clearasil bp acne treatment |
| Nyper B |
| BPO |
| EINECS 202-327-6 |
| Ins-928 |
| Nyper BMT |
| Cuticura acne cream |
| Lucidol 70 |
| Lucidol 78 |
| Stri-dex B.P |
| Oxy-L |
| component of Oxy-5 |
| Peroxyde de benzoyle [French] |
| component of Vanoxide |
| NSC671 |
| Xerac BP 5 |
| DTXSID6024591 |
| G 20 |
| Perossido di benzoile [Italian] |
| Clearasil benzoyl peroxide lotion |
| Cadet BPO 78W |
| Xerac BP 10 |
| Hydrous benzoyl peroxide |
| Abcure S-40-25 |
| Benzoylis peroxidum cum aqua |
| Benzoyl peroxide [USAN:USP] |
| DTXCID001072 |
| NSC675 |
| E-928 |
| CHEBI:82405 |
| NSC 675 |
| EC 202-327-6 |
| C14H10O4 |
| DUAC COMPONENT BENZOYL PEROXIDE |
| 2685-64-5 |
| ACANYA COMPONENT BENZOYL PEROXIDE |
| EPIDUO COMPONENT BENZOYL PEROXIDE |
| TWYNEO COMPONENT BENZOYL PEROXIDE |
| NCGC00159380-02 |
| NCGC00159380-05 |
| BENZOYL PEROXIDE COMPONENT OF DUAC |
| Benzoylperoxid (german) |
| W 75 |
| WLN: RVOOVR |
| Benzoyl peroxide (usan) |
| BENZACLIN COMPONENT BENZOYL PEROXIDE |
| BENZOYL PEROXIDE COMPONENT OF ACANYA |
| BENZOYL PEROXIDE COMPONENT OF EPIDUO |
| BENZOYL PEROXIDE COMPONENT OF TWYNEO |
| BENZAMYCIN COMPONENT BENZOYL PEROXIDE |
| Dibenzoylperoxid (german) |
| BENZOYL PEROXIDE COMPONENT OF BENZACLIN |
| BENZOYL PEROXIDE COMPONENT OF BENZAMYCIN |
| Novadelox (18% benzoyl peroxide, 78% calcium sulphate, 4% magnesium carbonate) |
| BENZOYL PEROXIDE (IARC) |
| BENZOYL PEROXIDE [IARC] |
| BENZOYL PEROXIDE (MART.) |
| BENZOYL PEROXIDE [MART.] |
| Androstan-17-one, 3-(acetyloxy)-5-bromo-6,19-epoxy-, (3b,5a,6b)- |
| BENZOYL PEROXIDE (USP IMPURITY) |
| BENZOYL PEROXIDE [USP IMPURITY] |
| CAS-94-36-0 |
| HYDROUS BENZOYL PEROXIDE (USP MONOGRAPH) |
| HYDROUS BENZOYL PEROXIDE [USP MONOGRAPH] |
| Benzoylperoksid |
| Thermaderm |
| Lucilite |
| Lucipal |
| Novadeiox |
| Periygel |
| Peroxyl |
| Presadox |
| bezoyl peroxide |
| Florox |
| Benzoyl peroide |
| Triaz |
| Luperco A |
| di-benzoylperoxide |
| dibenzoy lperoxide |
| dibenzoyl peroxyde |
| dibenzoyl-peroxide |
| Lucidol GS |
| Lucidol RM |
| Luperco AC |
| Luperco AFR |
| BzOOBz |
| bis benzoylperoxide |
| Bisbenzoyl peroxide |
| Cadox BTA |
| di-benzoyl peroxide |
| Perkadox 20S |
| Lucidol-78 |
| Luperox A98 |
| Peroxido de benzoilo |
| MFCD00003071 |
| BBPO |
| Bepio (TN) |
| Benzagel (Salt/Mix) |
| BENZOYL DIOXIDE |
| Luperco AFR-250 |
| Sulfoxyl (Salt/Mix) |
| SANPEROX BPO |
| CADET BPO |
| Cadet BPO-70W |
| Cadox BPO-W40 |
| Cadox BTW-50 |
| Benzoyl peroxide(usan) |
| NYPER BO |
| NYPER BS |
| NYPER BW |
| NYPER FF |
| NYPER NS |
| Benzoyl Peroxide [1] |
| Benzoyl Peroxide [2] |
| Benzoyl Peroxide [3] |
| Benzoyl Peroxide [4] |
| Benzoyl Peroxide [5] |
| Benzoyl Peroxide [6] |
| Benzoyl Peroxide [7] |
| Benzoyl Peroxide [8] |
| Benzoyl Peroxide [9] |
| DPO (CHRIS Code) |
| Benzoyl peroxide, USAN |
| NYPER FF-K |
| Xerac BP (Salt/Mix) |
| AcetOxyl 2.5 and 5 |
| Benzoyl peroxide (USP) |
| Diphenylperoxyanhydride # |
| SCHEMBL63 |
| TRIAZ;Dibenzoyl peroxide |
| Fostex BPO (Salt/Mix) |
| LUCIDOL 40E |
| LUCIDOL BW 50T |
| Aztec benzoyl peroxide-dry |
| LUCIDOL 98 |
| LUCIDOL BT 50 |
| LUCIDOL CH 50 |
| D04DXN |
| LUCIDOL S 50 |
| LUCIDOL W 40 |
| NYPER BMT 40 |
| TC 1 (PEROXIDE) |
| CADOX B 75W |
| Benoxyl (5 &10) Lotion |
| Benzoyl peroxide (JAN/USP) |
| MLS000028899 |
| BENZOYL PEROXIDE [MI] |
| BIDD:GT0840 |
| BENZOYL PEROXIDE (WET) |
| BENZOYL PEROXIDE [FCC] |
| BENZOYL PEROXIDE [JAN] |
| Aztec benzoyl peroxide-70-77 |
| SUPEROX 46-750 |
| BENZOYL PEROXIDE [HSDB] |
| BENZOYL PEROXIDE [INCI] |
| BENZOYL PEROXIDE [VANDF] |
| CHEMBL1200370 |
| Benzoyl peroxide, remainder water |
| Fostex BPO Bar, Gel, and Wash |
| BENZOYL PEROXIDE [WHO-DD] |
| component of Vanoxide (Salt/Mix) |
| HMS2092F22 |
| Pharmakon1600-01503004 |
| phenylcarbonyl benzenecarboperoxoate |
| NA 2085 (DOT) |
| UN 2085 (DOT) |
| IDP-126 component Benzoyl peroxide |
| Tox21_111619 |
| Benzoyl peroxide (Luperox(R) A75) |
| Benzoyl peroxide (Luperox(R) A98) |
| BR1012 |
| NA2085 |
| NA2087 |
| NA2088 |
| NA2090 |
| NSC758205 |
| UN2085 |
| UN2087 |
| UN2088 |
| UN2090 |
| Benzoyl peroxide (Luperox(R) A70S) |
| AKOS000120600 |
| benzenecarboperoxoic acid benzoyl ester |
| BENZOYL PEROXIDE [ORANGE BOOK] |
| Tox21_111619_1 |
| Benzoyl peroxide (Luperox(R) A75FP) |
| CCG-213090 |
| CS-T-05285 |
| DB09096 |
| LS-1962 |
| NSC-758205 |
| SC10147 |
| UN 2086 |
| UN 2088 |
| NCGC00159380-03 |
| NCGC00159380-04 |
| BP-21236 |
| E928 |
| HYDROUS BENZOYL PEROXIDE [WHO-IP] |
| SMR000058568 |
| SBI-0206719.P001 |
| B3152 |
| Benzoyl peroxide, blend in dibutyl phthalate |
| Benzoyl peroxide, blend in tricresyl phosphate |
| C19346 |
| D03093 |
| AB01562988_01 |
| Benzoyl peroxide, for synthesis, 72.0-80.0% |
| A844933 |
| Q411424 |
| SR-05000001817 |
| BENZOYLIS PEROXIDUM CUM AQUA [WHO-IP LATIN] |
| SR-05000001817-1 |
| BRD-K59986511-001-02-3 |
| Benzoyl peroxide, 97% (dry wt.), wet with 25% water |
| Benzoyl peroxide, SAJ first grade, >=98.0% dry basis |
| F0001-2260 |
| Luperox(R) A75, Benzoyl peroxide 75%, remainder water |
| Z104489074 |
| Luperox(R) A70S, Benzoyl peroxide, 70%, remainder water |
| Luperox(R) A75, Benzoyl peroxide, 75%, remainder water |
| Luperox(R) A98, Benzoyl peroxide, reagent grade, >=98% |
| BENZOYL PEROXIDE (SEE ALSO DMBA/TPA/BPO/MNNG (CAS NO. INIT/PROM)) |
| Benzoyl peroxide blend with dicyclohexyl phthalate, technical, ~50% (T) |
| Luperox(R) ATC50, Benzoyl peroxide, ~50 wt. % in tricresyl phosphate |
| InChI=1/C14H10O4/c15-13(11-7-3-1-4-8-11)17-18-14(16)12-9-5-2-6-10-12/h1-10 |
| Luperox(R) A75FP, Benzoyl peroxide, 75% remainder water, contains 25 wt. % water as stabilizer, 75% |
|
There are more than 10 synonyms. If you wish to see them all click here.
|