| 472-61-7 |
| Ovoester |
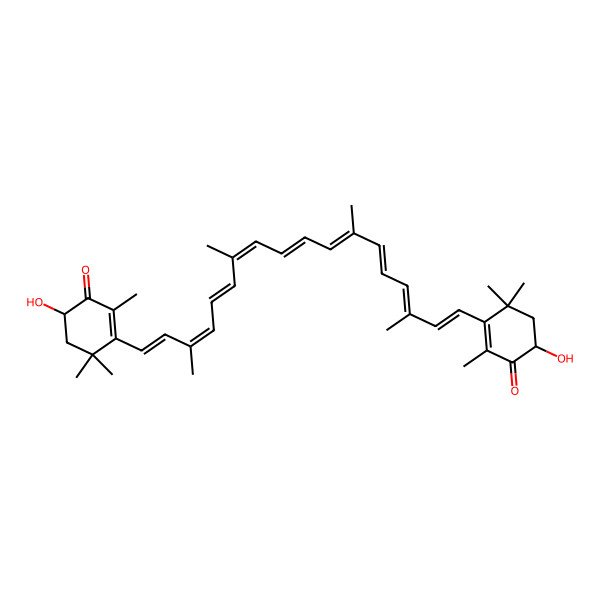
| all-trans-Astaxanthin |
| AstaREAL |
| 3,3'-Dihydroxy-beta,beta-carotene-4,4'-dione |
| Astaxanthine |
| BioAstin |
| (3S,3'S)-Astaxanthin |
| trans-Astaxanthin |
| Astaxanthin, (3S,3'S)- |
| (3S,3'S)-all-trans-Astaxanthin |
| 8XPW32PR7I |
| 3,3'-Dihydroxy-beta-carotene-4,4'-dione |
| (3S,3'S)-3,3'-Dihydroxy-beta,beta-carotene-4,4'-dione |
| CHEBI:40968 |
| NSC-635689 |
| (6S,6'S)-3,3'-((1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl)bis(6-hydroxy-2,4,4-trimethylcyclohex-2-enone) |
| .beta.,.beta.-Carotene-4,4'-dione, 3,3'-dihydroxy-, (3S,3'S)- |
| Algae Haematococcus pluvialis |
| Natupink |
| AstaXin |
| Carophyll Pink |
| Lucantin Pink |
| BioAstin oleoresin |
| (6S)-6-Hydroxy-3-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-[(4S)-4-hydroxy-2,6,6-trimethyl-3-oxo-1-cyclohexenyl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-2,4,4-trimethyl-1-cyclohex-2-enone |
| Astaxanthin (6CI) |
| Astaxanthin, all-trans- |
| (6S)-6-hydroxy-3-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-[(4S)-4-hydroxy-2,6,6-trimethyl-3-oxocyclohexen-1-yl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-2,4,4-trimethylcyclohex-2-en-1-one |
| UNII-8XPW32PR7I |
| astaxantin |
| CCRIS 7118 |
| HSDB 7468 |
| EINECS 207-451-4 |
| NSC 635689 |
| 3S,3'S-Astaxanthin |
| Astaxanthin, all-trans-, (3S,3'S)- |
| Astaxanthin, 5% active |
| ASTAXANTHIN [MI] |
| b-Carotene-4,4'-dione |
| ASTAXANTHIN [HSDB] |
| ASTAXANTHIN [INCI] |
| ASTAXANTHIN [VANDF] |
| beta-Carotene-4,4'-dione |
| ASTAXANTHIN [MART.] |
| SCHEMBL20047 |
| ASTAXANTHIN [USP-RS] |
| ASTAXANTHIN [WHO-DD] |
| E161j |
| CHEMBL1255871 |
| Astaxanthin, >=98% (HPLC) |
| E 161j |
| DTXSID00893777 |
| all-trans-(3S,3'S)-astaxanthin |
| 3,3'-Dihydroxy-beta,beta-carotene-4,4'-dione, (3S,3'S)- |
| HMS3885C08 |
| BCP05821 |
| HY-B2163 |
| LMPR01070263 |
| MFCD00672621 |
| s3834 |
| AKOS015841055 |
| AKOS015895756 |
| AC-8760 |
| BCP9000329 |
| CCG-270185 |
| DB06543 |
| all-trans-Astaxanthin, analytical standard |
| AS-14095 |
| CS-0020413 |
| C08580 |
| M01303 |
| 3,3'-dihydroxy-4,4'-diketo-beta,beta-carotene |
| A827177 |
| Q413740 |
| Q-200655 |
| 3,3'-DIHYDROXY-4,4'-DIKETO-.BETA.-CAROTENE |
| all-trans-3,3'-dihydroxy-b-Carotene-4,4'-dione (8CI) |
| .beta.-Carotene-4,4'-dione, 3,3'-dihydroxy-, all-trans- |
| 3-(4,6-Dimethyl-2-oxo-2H-pyrimidin-1-yl)-propionicacid |
| all-trans-3,3'-dihydroxy-beta-Carotene-4,4'-dione (8CI) |
| 3,3'-Dihydroxy-.beta.,.beta.-carotene-4,4'-dione, (3S,3'S)- |
| (3S,3'S)-3,3'-DIHYDROXY-.BETA.,.BETA.-CAROTENE-4,4'-DIONE |
| 3,3'-Dihydroxy-beta,beta-carotene-4,4'-dione;(S)-6-hydroxy-3-((1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-((S)-4-hydroxy-2,6,6-trimethyl-3-oxocyclohex-1-enyl)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl)-2,4,4-trimethylcyclohex-2-enone; |
|
There are more than 10 synonyms. If you wish to see them all click here.
|