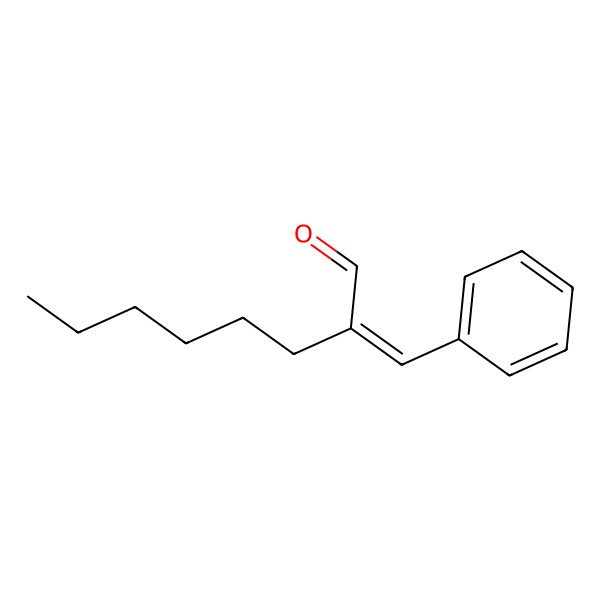
| 101-86-0 |
| (2E)-2-benzylideneoctanal |
| 2-Benzylideneoctanal |
| Hexyl cinnamic aldehyde |
| Hexylcinnamaldehyde |
| 165184-98-5 |
| (E)-2-benzylideneoctanal |
| alpha-Hexylcinnamic aldehyde |
| alpha-n-hexylcinnamaldehyde |
| (2E)-2-(phenylmethylidene)octanal |
| 2-Hexyl-3-phenyl-2-propenal |
| 2-(Phenylmethylene)octanal |
| CHEBI:55365 |
| Octanal, 2-(phenylmethylene)- |
| 2-Hexenyl cynnamaldehyde |
| alpha -hexylcinnamaldehyde |
| 2-(Phenylmethylidene)octanal |
| 2-Hexylcinnamaldehyde |
| UNII-E9947QRR9O |
| Hexylcinnamal |
| 2-[(E)-benzylidene]octanal |
| E9947QRR9O |
| alpha-Hexylcinnamyl aldehyde |
| alpha-Hexylcinnamaldehyde, (2E)- |
| alpha-Hexyl-beta-phenylacrolein |
| alpha-n-Hexyl-beta-phenylacrolein |
| .alpha.-Hexylcinnamaldehyde |
| EC 639-566-4 |
| WLN: VHY6 & U1R |
| DTXCID806684 |
| hexyl cinnamal |
| alpha-hexylkanelaldehyd |
| n-Hexyl cinnamaldehyde |
| a - hexylcinnamaldehyde |
| CAS-101-86-0 |
| alpha-hexyl cinnamaldehyde |
| 2-Hexyl-3-phenyl-propenal |
| .alpha.-Hexylcinnamic aldehyde |
| DTXSID4026684 |
| Hexylzimtaldehyd |
| .alpha.-n-Hexyl-.beta.-phenylacrolein |
| -hexylcinnamaldehyde |
| ?-Hexylcinnamaldehyde |
| alpha-hexylcinna-maldehyde |
| -Hexyl-3-phenyl-propenal |
| alfa-Hexyl Cinnam Aldehyde |
| Epitope ID:117426 |
| a-Hexylcinnamaldehyde, 8CI |
| 2-(phenylmethylene)-octanal |
| alpha -hexylcinnamic aldehyde |
| MLS002174256 |
| Cinnamaldehyde, alpha -hexyl- |
| SCHEMBL113170 |
| Hexyl Cinnamic Aldehyde Natural |
| CHEMBL1449245 |
| FEMA 2569 |
| (2E)-alpha-n-hexylcinnamaldehyde |
| 2-(Phenylmethylene)octanal, 9CI |
| DTXSID401020801 |
| HMS3039O14 |
| 2-HEXYL-(E)-CINNAMALDEHYDE |
| NSC46150 |
| alpha -N-hexyl-beta -phenylacrolein |
| Tox21_202301 |
| Tox21_300142 |
| BBL027629 |
| MFCD00006989 |
| NSC-46150 |
| NSC406799 |
| STK709222 |
| (2Z)-2-Hexyl-3-phenyl-2-propenal |
| AKOS015839664 |
| CS-W014834 |
| HY-W014118 |
| NSC-406799 |
| NCGC00090930-01 |
| NCGC00090930-02 |
| NCGC00254188-01 |
| NCGC00259850-01 |
| alpha -N-hexyl-alpha -hexylcinnamaldehyde |
| Octanal, 2-(phenylmethylene)-, (2E)- |
| SMR001261427 |
| VS-08571 |
| .ALPHA.-HEXYLCINNAMALDEHYDE, (2E)- |
| EN300-18426 |
| Hexylcinnamal 2000 microg/mL in Acetonitrile |
| OCTANAL, 2-(PHENYLMETHYLENE)-, (E)- |
| alpha-Hexylcinnamaldehyde, technical grade, 85% |
| alpha-Hexylcinnamaldehyde, >=95%, stabilized, FG |
| Q412025 |
| W-108898 |
| Z57936859 |
| 2-(phenylmethylene)octanal (.beta.-hexyl cinnamaldehyde) |
| alpha-Hexylcinnamaldehyde, analytical reference material |
| (E)-2-benzylideneoctanal, alpha-Hexyl Cinnamic Aldehyde, (2E)-2-(phenylmethylidene)octanal |
|
There are more than 10 synonyms. If you wish to see them all click here.
|