Aflatoxin G1
| Internal ID | 313482e3-ac4b-4776-be68-f14e2bdfd1c1 |
| Taxonomy | Phenylpropanoids and polyketides > Coumarins and derivatives > Furanocoumarins > Aflatoxins > Difurocoumarolactones |
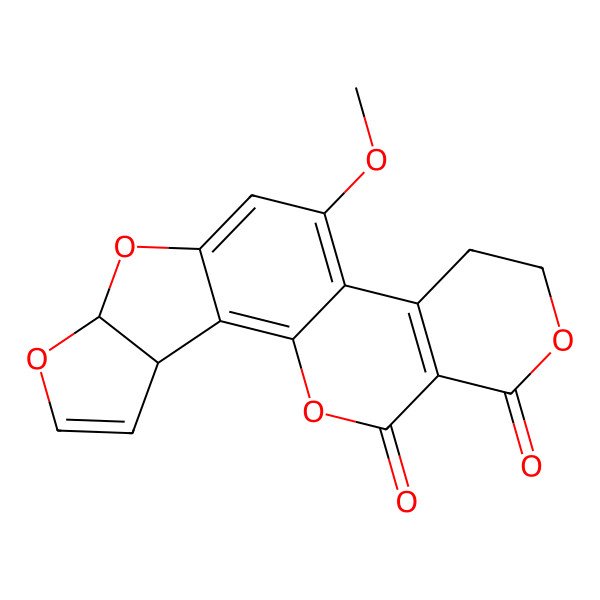
| IUPAC Name | 11-methoxy-6,8,16,20-tetraoxapentacyclo[10.8.0.02,9.03,7.013,18]icosa-1,4,9,11,13(18)-pentaene-17,19-dione |
| SMILES (Canonical) | COC1=C2C3=C(C(=O)OCC3)C(=O)OC2=C4C5C=COC5OC4=C1 |
| SMILES (Isomeric) | COC1=C2C3=C(C(=O)OCC3)C(=O)OC2=C4C5C=COC5OC4=C1 |
| InChI | InChI=1S/C17H12O7/c1-20-9-6-10-12(8-3-5-22-17(8)23-10)14-11(9)7-2-4-21-15(18)13(7)16(19)24-14/h3,5-6,8,17H,2,4H2,1H3 |
| InChI Key | XWIYFDMXXLINPU-UHFFFAOYSA-N |
| Popularity | 246 references in papers |
| Molecular Formula | C17H12O7 |
| Molecular Weight | 328.27 g/mol |
| Exact Mass | 328.05830272 g/mol |
| Topological Polar Surface Area (TPSA) | 80.30 Ų |
| XlogP | 1.80 |
| Atomic LogP (AlogP) | 1.86 |
| H-Bond Acceptor | 7 |
| H-Bond Donor | 0 |
| Rotatable Bonds | 1 |
| Aflatoxin |
| 1165-39-5 |
| 1402-68-2 |
| Aflatoxin G |
| Aflatoxin, crude |
| Aflatoxin G1-d3 |
| 11-methoxy-6,8,16,20-tetraoxapentacyclo[10.8.0.02,9.03,7.013,18]icosa-1,4,9,11,13(18)-pentaene-17,19-dione |
| 5-(methyloxy)-3,4,7a,10a-tetrahydro-1H,12H-furo[3',2':4,5]furo[2,3-h]pyrano[3,4-c]chromene-1,12-dione |
| AflatoxinG |
| HSDB 3411 |
| There are more than 10 synonyms. If you wish to see them all click here. |
| Target | Value | Probability (raw) | Probability (%) |
|---|---|---|---|
| Human Intestinal Absorption | + | 0.9926 | 99.26% |
| Caco-2 | + | 0.5413 | 54.13% |
| Blood Brain Barrier | + | 0.6000 | 60.00% |
| Human oral bioavailability | - | 0.6000 | 60.00% |
| Subcellular localzation | Mitochondria | 0.8603 | 86.03% |
| OATP2B1 inhibitior | - | 1.0000 | 100.00% |
| OATP1B1 inhibitior | + | 0.9154 | 91.54% |
| OATP1B3 inhibitior | + | 0.9797 | 97.97% |
| MATE1 inhibitior | - | 0.7800 | 78.00% |
| OCT2 inhibitior | - | 0.8250 | 82.50% |
| BSEP inhibitior | + | 0.6260 | 62.60% |
| P-glycoprotein inhibitior | - | 0.4814 | 48.14% |
| P-glycoprotein substrate | - | 0.8569 | 85.69% |
| CYP3A4 substrate | + | 0.6024 | 60.24% |
| CYP2C9 substrate | - | 0.5841 | 58.41% |
| CYP2D6 substrate | - | 0.8415 | 84.15% |
| CYP3A4 inhibition | - | 0.5165 | 51.65% |
| CYP2C9 inhibition | + | 0.8106 | 81.06% |
| CYP2C19 inhibition | + | 0.8180 | 81.80% |
| CYP2D6 inhibition | + | 0.5087 | 50.87% |
| CYP1A2 inhibition | + | 0.8582 | 85.82% |
| CYP2C8 inhibition | + | 0.5207 | 52.07% |
| CYP inhibitory promiscuity | + | 0.6781 | 67.81% |
| UGT catelyzed | - | 0.0000 | 0.00% |
| Carcinogenicity (binary) | - | 0.9600 | 96.00% |
| Carcinogenicity (trinary) | Danger | 0.5779 | 57.79% |
| Eye corrosion | - | 0.9572 | 95.72% |
| Eye irritation | - | 0.8596 | 85.96% |
| Skin irritation | - | 0.8123 | 81.23% |
| Skin corrosion | - | 0.9596 | 95.96% |
| Ames mutagenesis | + | 0.9800 | 98.00% |
| Human Ether-a-go-go-Related Gene inhibition | - | 0.6921 | 69.21% |
| Micronuclear | - | 0.5467 | 54.67% |
| Hepatotoxicity | + | 0.8125 | 81.25% |
| skin sensitisation | - | 0.6993 | 69.93% |
| Respiratory toxicity | + | 0.5889 | 58.89% |
| Reproductive toxicity | + | 0.8556 | 85.56% |
| Mitochondrial toxicity | - | 0.5000 | 50.00% |
| Nephrotoxicity | - | 0.7289 | 72.89% |
| Acute Oral Toxicity (c) | I | 0.6402 | 64.02% |
| Estrogen receptor binding | + | 0.8319 | 83.19% |
| Androgen receptor binding | + | 0.7621 | 76.21% |
| Thyroid receptor binding | - | 0.6396 | 63.96% |
| Glucocorticoid receptor binding | + | 0.7810 | 78.10% |
| Aromatase binding | + | 0.6745 | 67.45% |
| PPAR gamma | + | 0.8205 | 82.05% |
| Honey bee toxicity | - | 0.7281 | 72.81% |
| Biodegradation | - | 0.8750 | 87.50% |
| Crustacea aquatic toxicity | + | 0.5900 | 59.00% |
| Fish aquatic toxicity | + | 0.9117 | 91.17% |
Proven Targets:
| CHEMBL ID | UniProt ID | Name | Min activity | Assay type | Source |
|---|---|---|---|---|---|
| No proven targets yet! | |||||
Predicted Targets (via Super-PRED):
| CHEMBL ID | UniProt ID | Name | Probability | Model accuracy |
|---|---|---|---|---|
| CHEMBL5619 | P27695 | DNA-(apurinic or apyrimidinic site) lyase | 98.01% | 91.11% |
| CHEMBL4040 | P28482 | MAP kinase ERK2 | 96.27% | 83.82% |
| CHEMBL3108638 | O15164 | Transcription intermediary factor 1-alpha | 95.32% | 95.56% |
| CHEMBL2635 | P51452 | Dual specificity protein phosphatase 3 | 92.56% | 94.00% |
| CHEMBL4261 | Q16665 | Hypoxia-inducible factor 1 alpha | 92.01% | 85.14% |
| CHEMBL1293249 | Q13887 | Kruppel-like factor 5 | 90.65% | 86.33% |
| CHEMBL2535 | P11166 | Glucose transporter | 89.50% | 98.75% |
| CHEMBL1806 | P11388 | DNA topoisomerase II alpha | 88.00% | 89.00% |
| CHEMBL2373 | P21730 | C5a anaphylatoxin chemotactic receptor | 86.96% | 92.62% |
| CHEMBL2581 | P07339 | Cathepsin D | 85.91% | 98.95% |
| CHEMBL4478 | Q00975 | Voltage-gated N-type calcium channel alpha-1B subunit | 83.95% | 97.14% |
| CHEMBL5608 | Q16288 | NT-3 growth factor receptor | 82.90% | 95.89% |
| CHEMBL2094127 | P06493 | Cyclin-dependent kinase 1/cyclin B | 82.73% | 96.00% |
| CHEMBL4203 | Q9HAZ1 | Dual specificity protein kinase CLK4 | 82.06% | 94.45% |
| CHEMBL4481 | P35228 | Nitric oxide synthase, inducible | 81.40% | 94.80% |
| CHEMBL5697 | Q9GZT9 | Egl nine homolog 1 | 81.31% | 93.40% |
| CHEMBL3038477 | P67870 | Casein kinase II alpha/beta | 80.80% | 99.23% |
| PubChem | 14421 |
| LOTUS | LTS0104763 |
| wikiData | Q59260340 |