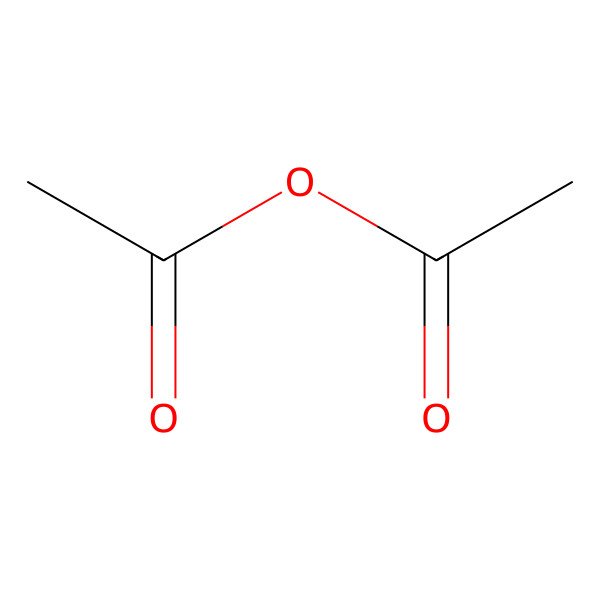
| Acetyl acetate |
| 108-24-7 |
| Acetanhydride |
| Ethanoic anhydride |
| Acetyl anhydride |
| Acetic acid, anhydride |
| Acetyl oxide |
| acetic acid anhydride |
| Acetic oxide |
| Acetyl ether |
| Anhydride acetique |
| Anidride acetica |
| Octowy bezwodnik |
| Azijnzuuranhydride |
| Essigsaeureanhydrid |
| Ethanoic anhydrate |
| Anhydrid kyseliny octove |
| Ac2O |
| (CH3CO)2O |
| Caswell No. 003A |
| ethanoyl ethanoate |
| HSDB 233 |
| CCRIS 688 |
| Acetic acid, 1,1'-anhydride |
| Octowy bezwodnik [Polish] |
| Azijnzuuranhydride [Dutch] |
| Anidride acetica [Italian] |
| Anhydride acetique [French] |
| EINECS 203-564-8 |
| Essigsaeureanhydrid [German] |
| UNII-2E48G1QI9Q |
| EPA Pesticide Chemical Code 044007 |
| BRN 0385737 |
| 2E48G1QI9Q |
| DTXSID0024395 |
| CHEBI:36610 |
| Anhydrid kyseliny octove [Czech] |
| (MeCO)2O |
| UN1715 |
| DTXCID704395 |
| EC 203-564-8 |
| 4-02-00-00386 (Beilstein Handbook Reference) |
| Acetic anhydride-1,1'-13C2,d6 |
| ACETIC ANHYDRIDE-13C4, D6 |
| Acetic anhydride [UN1715] [Corrosive] |
| ACETIC ANHYDRIDE (1,1'-13C2) |
| ACETIC ANHYDRIDE (II) |
| ACETIC ANHYDRIDE [II] |
| MFCD00008705 |
| 285977-77-7 |
| 285977-78-8 |
| acetanhydrid |
| acetic anhydrid |
| actic anhydride |
| diacetyl oxide |
| acetic-anhydride |
| Eddiksyreanhydrid |
| Anhydride actique |
| acetic, anhydride |
| AcOAc |
| Acetyltrimethyl-Silane |
| acetic acetic anhydride |
| ACA (CHRIS Code) |
| acetic acid acetyl ester |
| CAPPING REAGENT A |
| Acetyl ether Acetyl oxide |
| SCHEMBL523 |
| Acetic anhydride ACS grade |
| acetic acid-acetic anhydride |
| (Ac)2O |
| Acetic anhydride, >=99% |
| Acetic anhydride, 99.5% |
| Pesticide Code: 044007 |
| Acetic Anhydride Reagent ACS |
| MLS002454384 |
| ACETIC ANHYDRIDE [MI] |
| ACETIC ANHYDRIDE [HSDB] |
| CHEMBL1305819 |
| HMS3039J03 |
| BCP20665 |
| Acetic anhydride, JIS special grade |
| Tox21_200278 |
| LS-456 |
| NA1715 |
| STL264203 |
| AKOS015915487 |
| UN 1715 |
| NCGC00091802-01 |
| NCGC00091802-02 |
| NCGC00257832-01 |
| Acetic anhydride, reagent grade, >=98% |
| BP-24367 |
| BP-31054 |
| CAS-108-24-7 |
| SMR001372001 |
| Acetic anhydride [UN1715] [Corrosive] |
| Acetic anhydride, ACS reagent, >=98.0% |
| Acetic anhydride, ReagentPlus(R), >=99% |
| Acetic anhydride, p.a., ACS reagent, 97.0% |
| Acetic anhydride, SAJ first grade, >=93.0% |
| A801828 |
| Acetic anhydride, Lonza quality, >=99.5% (GC) |
| Q407775 |
| Acetic anhydride, for GC derivatization, >=99.0% |
| InChI=1/C4H6O3/c1-3(5)7-4(2)6/h1-2H |
| Acetic anhydride, p.a., ACS reagent, reag. ISO, reag. Ph. Eur., 99.0% |
| Acetic anhydride, puriss. p.a., ACS reagent, reag. ISO, reag. Ph. Eur., >=99% (GC) |
|
There are more than 10 synonyms. If you wish to see them all click here.
|