| 652-37-9 |
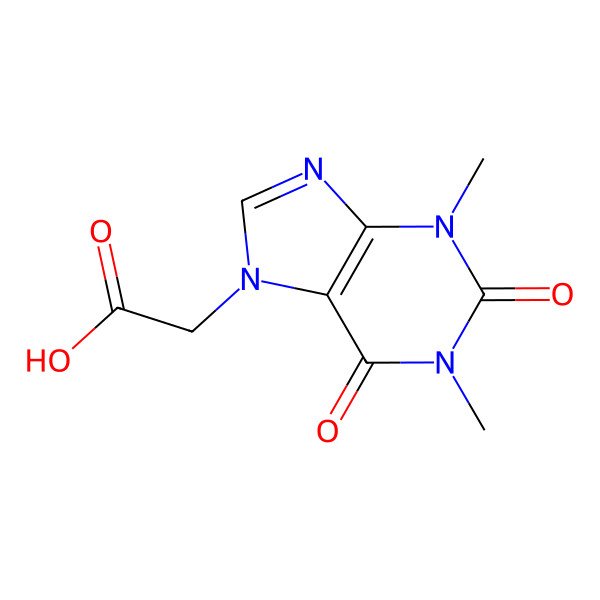
| Theophylline-7-acetic acid |
| Acephylline |
| Theophyllineacetic acid |
| Carboxymethyltheophylline |
| Theophyllin-7-ylacetic acid |
| 7-(Carboxymethyl)theophylline |
| 7-Theophyllineacetic acid |
| 7-Theophyllinylacetic acid |
| 7-Theophyllinessigsaeure |
| 7-Theophyllinylessigsaeure |
| 1,3-Dimethylxanthine-7-acetic acid |
| NSC 52996 |
| (1,3-Dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-7H-purin-7-yl)acetic acid |
| 2-(1,3-dimethyl-2,6-dioxopurin-7-yl)acetic acid |
| Purine-7-acetic acid, 1,2,3,6-tetrahydro-1,3-dimethyl-2,6-dioxo- |
| caffeine carboxylic acid |
| 2-(1,3-Dimethyl-2,6-dioxo-2,3-dihydro-1H-purin-7(6H)-yl)acetic acid |
| EINECS 211-490-2 |
| MFCD00022832 |
| NSC-52996 |
| Doxophylline metabolite m2 |
| UNII-M494UE2YEP |
| aethaphyllinum |
| BRN 0279221 |
| M494UE2YEP |
| 7H-Purine-7-acetic acid, 1,2,3,6-tetrahydro-1,3-dimethyl-2,6-dioxo- |
| Acide theophylline-ethanoique-7 [French] |
| CHEMBL70246 |
| Acide theophylline-ethanoique-7 |
| DTXSID6057796 |
| 1,2,3,6-Tetrahydro-1,3-dimethyl-2,6-dioxopurine-7-acetic acid |
| ACEFYLLINE PIPERAZINE |
| Acepifylline |
| Epicophylline |
| 2-(1,3-Dimethyl-2,6-Dioxo-2,3,6,7-Tetrahydro-1H-Purin-7-Yl)Acetic Acid |
| 2-(1,3-Dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-7H-purin-7-yl)acetic acid |
| EC 211-490-2 |
| 2-(p-Chlorphenoxy)-2-methylpropyl 1,2,3,6-tetrahydro-1,3-dimethyl-2,6-dioxopurine-7-acetate |
| 5-26-14-00088 (Beilstein Handbook Reference) |
| Acefylline piperazine salt |
| Piperazine theophylline ethanoate |
| acetyloxytheophylline |
| Aminodal (Salt/Mix) |
| Theophylline-acetic acid |
| ACEFYLLINE [MI] |
| Acepifylline (Salt/Mix) |
| theophylline 7-acetic acid |
| ACEFYLLINE [WHO-DD] |
| Oprea1_398884 |
| MLS001032052 |
| SCHEMBL308514 |
| LCZC2430 |
| DTXCID7031585 |
| CHEBI:94615 |
| HMS3714E15 |
| HMS3885C16 |
| Acefylline sodium salt (Salt/Mix) |
| HY-B1505 |
| NSC52996 |
| Tox21_113765 |
| BBL027883 |
| BDBM50113248 |
| STK801809 |
| AKOS000120359 |
| CAFFEINE CARBOXYLIC ACID [INCI] |
| CCG-106578 |
| CS-7963 |
| DB13573 |
| NCGC00253638-01 |
| CAS-652-37-9 |
| SMR000718632 |
| TS-00694 |
| LS-126434 |
| FT-0632754 |
| S3988 |
| T2941 |
| EN300-20362 |
| Theophylline-7-acetic acid, >=99.0% (T) |
| A16822 |
| D82360 |
| A835035 |
| AF-684/00246037 |
| SR-01000898401 |
| 2-(1,3-dimethyl-2,6-dioxo-7-purinyl)acetic acid |
| Q-201818 |
| Q4673059 |
| SR-01000898401-2 |
| F0849-4607 |
| Z104477882 |
| Acefylline;Theophyllineacetic acid; Theophylline-7-acetic acid |
| Theophilline-7-acetic acid (1,3-Dimethylxantine-7-acetic acid) |
| 7H-Purine-7-acetic acid,2,3,6-tetrahydro-1,3-dimethyl-2,6-dioxo- |
| Purine-7-acetic acid,2,3,6-tetrahydro-1,3-dimethyl-2,6-dioxo- |
| (1,3-Dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-7H-purin-7-yl)acetic acid # |
| (1,3-Dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-purin-7-yl)-acetic acid |
| 1,2,3,6-tetrahydro-1,3-dimethyl-2,6-dioxo-7h-purine-7-acetic acid |
| 1,2,3,6-TETRAHYDRO-1,3-DIMETHYL-2,6-DIOXOPURINE-7-ACETIC A |
| 2-(1,3-Dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-7H-purin-7-yl)aceticacid |
| 52-37-9 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|