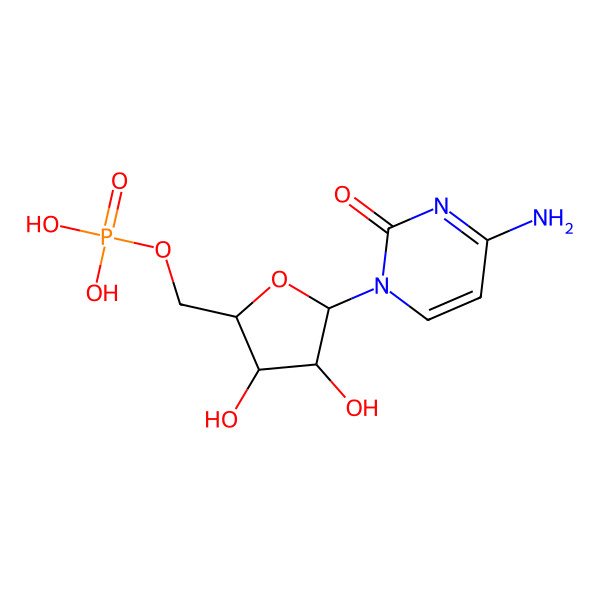
| Cytidine 5'-monophosphate |
| 63-37-6 |
| cytidylic acid |
| Cytidine monophosphate |
| Cytidine-5'-monophosphate |
| 5'-CMP |
| cytidylate |
| Cytidine 5'-phosphate |
| CMP (nucleotide) |
| Cytidine 5'-phosphoric acid |
| Cytidine 5'-monophosphoric acid |
| ((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl dihydrogen phosphate |
| Cytidine 5'-(dihydrogenphosphate) |
| CMP |
| cytidine-P |
| cytidine-phosphate |
| Cytidine 3'-(dihydrogen phosphate) |
| 1beta-Ribofuranosylcytosine 5'-phosphate, d- |
| C-5'-P |
| CHEBI:17361 |
| MFCD00006544 |
| Cytidine 5'-monophosphate, free acid |
| CHEMBL307679 |
| [(2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl dihydrogen phosphate |
| DTXSID50889322 |
| 5'-Cytidylic acid (8CI,9CI) |
| [(2R,3S,4R,5R)-5-(4-amino-2-oxo-pyrimidin-1-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl]methyl dihydrogen phosphate |
| {[(2R,3S,4R,5R)-5-(4-amino-2-oxo-1,2-dihydropyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}phosphonic acid |
| Cytidine, mono(dihydrogen phosphate) (ester) |
| F469818O25 |
| 2(1H)-pyrimidinone, 4-amino-1-(5-O-phosphono-beta-D-ribofuranosyl)- |
| Cytidine-5' Monophosphate |
| cytidine-monophosphate |
| 5'-Cytidylate monophosphate |
| C5P |
| 5'-CYTIDYLIC ACID (USP-RS) |
| 5'-CYTIDYLIC ACID [USP-RS] |
| C9H14N3O8P |
| EINECS 200-556-6 |
| Cytidine 5 -monophosphate |
| BRN 0046982 |
| 5-cytidylate |
| 5-Cytidylic acid |
| UNII-F469818O25 |
| cytidine mono phosphate |
| CytidylsA currencyure a |
| cytidine 5''-phosphate |
| Cytidine 5'-phosphorate |
| 5'-CYTIDYLICACID |
| bmse000311 |
| Epitope ID:196257 |
| Cytidine 5'-monophosphorate |
| 3H-Cytidin-5'-monophosphat |
| SCHEMBL16189 |
| 4-25-00-03673 (Beilstein Handbook Reference) |
| CYTIDYLIC ACID [WHO-DD] |
| CYTOSINE 5'-MONOPHOSPHATE |
| 5'-CYTIDYLIC ACID [FCC] |
| DTXCID701028585 |
| Cytidine mono(dihydrogen phosphate) |
| CYTIDINE-5''-MONOPHOSPHATE |
| AMY30020 |
| BDBM50310540 |
| s5374 |
| AKOS015854843 |
| AKOS015888565 |
| CCG-267735 |
| CS-W009878 |
| DB03403 |
| HY-W009162 |
| AC-32139 |
| AS-56853 |
| BP-58604 |
| C25 |
| SY030157 |
| 5,(S) |
| C1675 |
| C00055 |
| W18546 |
| EN300-7376706 |
| A820633 |
| Q412622 |
| Cytidine 5 inverted exclamation mark -Monophosphate |
| Q-101433 |
| B755C4F3-3D29-45E0-9432-16F902ABCFE1 |
| 5'-Cytidylic acid; Cytidine monophosphate, Cytidine 5'-phosphate |
| 5'-Cytidylic acid, United States Pharmacopeia (USP) Reference Standard |
| Cytidine 5'-monophosphate, Sigma Grade, >=99% (HPLC), synthetic, powder |
| ((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl)methyl dihydrogen phosphate |
| FN5 |
| Phosphoric acid mono-[5-(4-amino-2-oxo-2H-pyrimidin-1-yl)-3,4-dihydroxy-tetrahydro-furan-2-ylmethyl] ester |
|
There are more than 10 synonyms. If you wish to see them all click here.
|