| 137-00-8 |
| sulfurol |
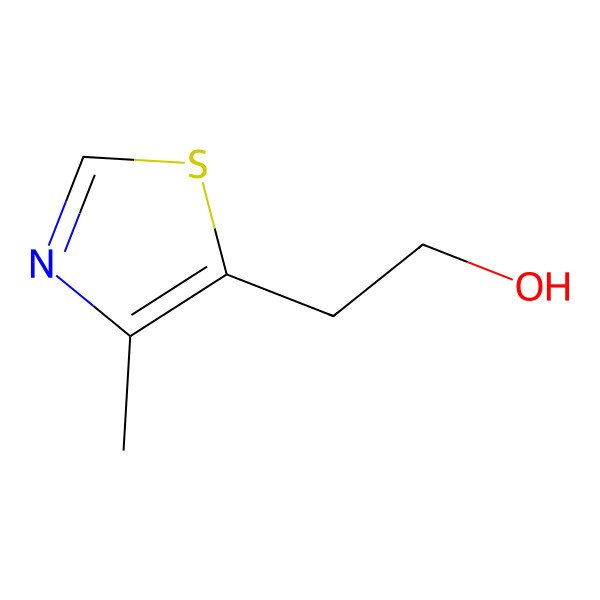
| 5-(2-Hydroxyethyl)-4-methylthiazole |
| Hemineurine |
| 2-(4-Methylthiazol-5-yl)ethanol |
| 5-Thiazoleethanol, 4-methyl- |
| 2-(4-Methyl-1,3-thiazol-5-yl)ethanol |
| 4-Methyl-5-thiazolethanol |
| 4-Methyl-5-(2-hydroxyethyl)thiazole |
| 4-Methyl-5-thiazolylethanol |
| 2-(4-Methylthiazole-5-yl)ethanol |
| Thiamine thiazole |
| Thiamine thiozole |
| 4-Methyl-5-hydroxethylthiazole |
| 4-Methyl-5-hydroxyethylthiazole |
| MHT (VAN) |
| 2-(4-METHYL-THIAZOL-5-YL)-ETHANOL |
| 2-(4-Methyl-1,3-Thiazol-5-Yl)Ethan-1-Ol |
| 5-(Hydroxyethyl)-4-methylthiazole |
| 4-methyl-5-thiazole ethanol |
| 5-(beta-Hydroxyethyl)-4-methylthiazole |
| FEMA No. 3204 |
| MHT |
| CHEBI:17957 |
| 2-(4-methyl-5-thiazolyl)ethanol |
| NSC 23262 |
| 4-methyl-5-(2-hydroxyethyl)-thiazole |
| 4-methyl-5-(beta-hydroxyethyl)thiazole |
| 4-Methyl-5-(2'-hydroxyethyl)-thiazole |
| 4-Methyl-5-(.beta.-hydroxyethyl)thiazole |
| 3XYV4I47I8 |
| CHEMBL1236482 |
| DTXSID3044382 |
| 4-METHYLTHIAZOL-5YLETHANOL |
| NSC-23262 |
| NSC-41831 |
| HET |
| Thiazole, 5-(2-hydroxyethyl)-4-methyl |
| TZE |
| UNII-3XYV4I47I8 |
| EINECS 205-272-6 |
| MFCD00005339 |
| NSC 41831 |
| Clomethiazole Impurity C |
| AI3-23391 |
| bmse000355 |
| 4-METHYL-5-(beta-HYDROXYETHYL)-THIAZOLE |
| 4-methyl-5-thiazole-ethanol |
| SCHEMBL259480 |
| DTXCID1024382 |
| FEMA 3204 |
| 4-Methyl-5-thiazoleethanol, 9CI |
| 4-Methyl-5-thiazoleethanol, 98% |
| 2-(4-methylthiazol-5-yl) ethanol |
| 2-(4-methylthiazol-5-yl)-ethanol |
| NSC23262 |
| NSC41831 |
| STR05522 |
| Thiazole, 4-methyl-5-hydroxyethyl- |
| Tox21_302187 |
| BDBM50016817 |
| AKOS000119411 |
| 4-Metyl-5-(beta-hydroxyethyl)thiazole |
| 5-(2-hydroxyethyl)-4-methyl-thiazole |
| AB00407 |
| AC-7852 |
| CS-W016411 |
| DB02969 |
| HY-W015695 |
| PS-4466 |
| 4-METHYL-5-HYDROXYETHYL THIAZOLE |
| 4-METHYL-5-THIAZOLEETHANOL [MI] |
| NCGC00257545-01 |
| CAS-137-00-8 |
| PD007492 |
| 4-METHYL-5-THIAZOLEETHANOL [FHFI] |
| 4-Methyl-5-thiazoleethanol, >=98%, FG |
| 4-METHYL-5-THIAZOLE ETHANOL [FCC] |
| 5-(2-Hydroxyethyl)-4-methylthiazole; MHT |
| 2-(4-Methyl-1,3-thiazol-5-yl)ethanol # |
| 5-(2-hydroxyethyl)-4-methyl-1,3-thiazole |
| AM20080272 |
| FT-0608672 |
| H0527 |
| EN300-20333 |
| 4-Methyl-5-thiazoleethanol, analytical standard |
| C04294 |
| D77790 |
| 5-(2-Hydroxyethyl)-4-methylthiazole (sulfurol) |
| 5-(.BETA.-HYDROXYETHYL)-4-METHYLTHIAZOLE |
| Q-100145 |
| Q27093940 |
| Z104477768 |
| 48114B6F-A59B-4E5B-82B1-38B17D2A0B5A |
| InChI=1/C6H9NOS/c1-5-6(2-3-8)9-4-7-5/h4,8H,2-3H2,1H |
| 4-methyl-5-thiazoleethanol; sulfurol; 4-methyl-5-(2'-hydroxyethyl)-thiazole; 4-methyl-5-hydroxyethylthiazole; 5-(2-hydroxyethyl)-4-methylthiazole; 4-methyl-5-beta-hydroxyethyl thiazole |
| 860175-16-2 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|