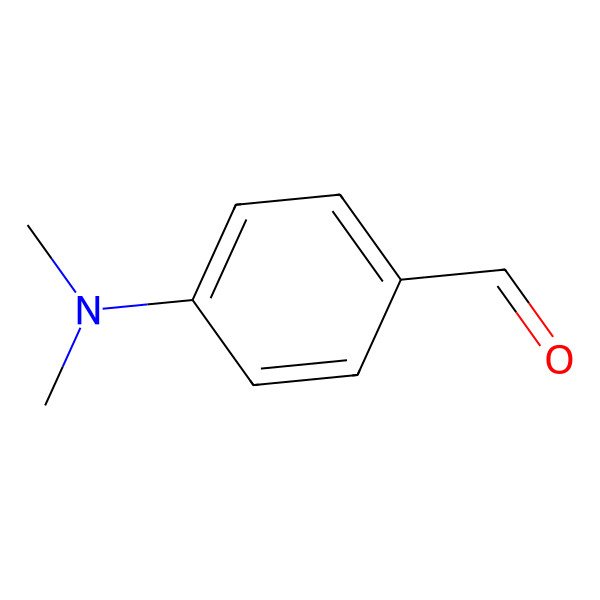
| 100-10-7 |
| 4-Dimethylaminobenzaldehyde |
| p-Dimethylaminobenzaldehyde |
| Ehrlich's reagent |
| Benzaldehyde, 4-(dimethylamino)- |
| p-Formyldimethylaniline |
| p-(Dimethylamino)benzaldehyde |
| p-Formyl-N,N-dimethylaniline |
| 4-Dimethylaminobenzenecarbonal |
| N,N-Dimethyl-p-aminobenzaldehyde |
| p-(N,N-Dimethylamino)benzaldehyde |
| para-Dimethylaminobenzaldehyde |
| Reagens ehrlichovo |
| Benzaldehyde, p-(dimethylamino)- |
| MFCD00003381 |
| NSC 5517 |
| Reagens ehrlichovo [Czech] |
| 4-dimethylamino-benzaldehyde |
| N,N-Dimethyl-4-formylaniline |
| 4-N,N-Dimethylaminobenzaldehyde |
| p-DAB |
| 4-N,N-DIMETHYLBENZALDEHYDE |
| 4-dimethylaminobenzaldehyd |
| EINECS 202-819-0 |
| UNII-V7E88PR1YB |
| 4-dimethylamino benzaldehyde |
| V7E88PR1YB |
| AI3-15337 |
| CHEMBL3188333 |
| DTXSID5021835 |
| N,N-Dimethyl-4-aminobenzaldehyde |
| NSC-5517 |
| Ehrlich's Reagent |
| Ehrlichs reagent |
| Kovac's reagent for indoles, for microbiology |
| P-Dimethgl anuinotenzaldehyde |
| 4-Formyl-N,N-dimethylaniline |
| hydrazine reagent |
| 4-formyl-N |
| 2v6n |
| 4-(N,N-DIMETHYLAMINO)BENZALDEHYDE |
| p-dimethylaminobenzaldehyd |
| p-dimethylamino benzaldehyde |
| Para-dimehtylaminobenzaldehyde |
| SCHEMBL61517 |
| 4-Formyl-N,N-dimethylanilin |
| Benzaldehyde, p-dimethylamino- |
| p-(dimethylamino) benzaldehyde |
| 4-(dimethylamino) benzaldehyde |
| 4-(dimethylamino)-benzaldehyde |
| DTXCID601835 |
| Dimethylamino)benzaldehyde, p-( |
| P-Dimethylaminobenzaldehyde,(S) |
| 4-(Dimethylamino)benzenecarbonal |
| WLN: VHR DN1 & 1 |
| CHEBI:91114 |
| 4-N,N-dimethylamino benzaldehyde |
| NSC5517 |
| para-N,N-dimethylaminobenzaldehyde |
| BCP30263 |
| HY-Y0015 |
| DIMETHYLAMINOBENZALDEHYDE, P- |
| Tox21_300707 |
| 4-(Dimethylamino)benzaldehyde, 98% |
| 4-DIMETHYL-AMINO-BENZALDEHYDE |
| BDBM50101990 |
| STK199263 |
| AKOS000118908 |
| CS-W020083 |
| P-DIMETHYLAMINOBENZALDEHYDE [MI] |
| NCGC00184048-01 |
| NCGC00184048-02 |
| NCGC00184048-03 |
| NCGC00184048-04 |
| NCGC00184048-05 |
| NCGC00254615-01 |
| AS-10767 |
| CAS-100-10-7 |
| LS-25022 |
| AM20060801 |
| D0645 |
| D1495 |
| FT-0618357 |
| p-Dimethylaminobenzaldehyde;Ehrlich's reagent |
| 4-(Dimethylamino)benzaldehyde A.C.S. reagent |
| EN300-18036 |
| 4-(Dimethylamino)benzaldehyde, p.a., 99.0% |
| W18656 |
| AB01333894-02 |
| 4-(Dimethylamino)benzaldehyde, ACS reagent, 99% |
| A800043 |
| AG-205/03202034 |
| J-200061 |
| Q1225737 |
| Z57127528 |
| F2190-0620 |
| 4-(Dimethylamino)benzaldehyde, JIS special grade, >=99.0% |
| 4-(Dimethylamino)benzaldehyde, Vetec(TM) reagent grade, 98% |
| InChI=1/C9H11NO/c1-10(2)9-5-3-8(7-11)4-6-9/h3-7H,1-2H |
| 4-(Dimethylamino)benzaldehyde, puriss. p.a., Reag. Ph. Eur., >=99% (perchloric acid titration) |
| 4-(Dimethylamino)benzaldehyde, suitable for histochemical demonstration of nitro blue tetrazolium reduction in neutrophils |
| 4-N |
| p-N |
|
There are more than 10 synonyms. If you wish to see them all click here.
|