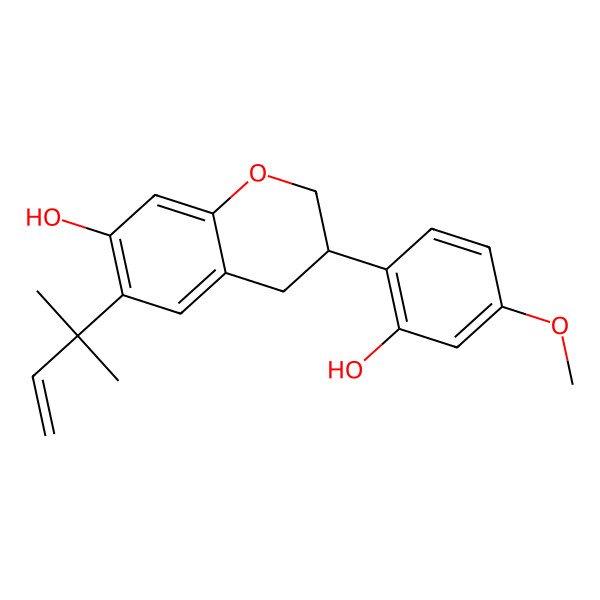
(3R)-3-(2-hydroxy-4-methoxyphenyl)-6-(2-methylbut-3-en-2-yl)-3,4-dihydro-2H-chromen-7-ol
| Internal ID | 0b95cac5-91fe-43ca-a7f9-6b72ef68616a |
| Taxonomy | Phenylpropanoids and polyketides > Isoflavonoids > O-methylated isoflavonoids > 4-O-methylated isoflavonoids |
| IUPAC Name | (3R)-3-(2-hydroxy-4-methoxyphenyl)-6-(2-methylbut-3-en-2-yl)-3,4-dihydro-2H-chromen-7-ol |
| SMILES (Canonical) | CC(C)(C=C)C1=C(C=C2C(=C1)CC(CO2)C3=C(C=C(C=C3)OC)O)O |
| SMILES (Isomeric) | CC(C)(C=C)C1=C(C=C2C(=C1)C[C@@H](CO2)C3=C(C=C(C=C3)OC)O)O |
| InChI | InChI=1S/C21H24O4/c1-5-21(2,3)17-9-13-8-14(12-25-20(13)11-19(17)23)16-7-6-15(24-4)10-18(16)22/h5-7,9-11,14,22-23H,1,8,12H2,2-4H3/t14-/m0/s1 |
| InChI Key | FSGITBYHHHOHAL-AWEZNQCLSA-N |
| Popularity | 0 references in papers |
| Molecular Formula | C21H24O4 |
| Molecular Weight | 340.40 g/mol |
| Exact Mass | 340.16745924 g/mol |
| Topological Polar Surface Area (TPSA) | 58.90 Ų |
| XlogP | 4.90 |
| There are no found synonyms. |
| Target | Value | Probability (raw) | Probability (%) |
|---|---|---|---|
| No predicted properties yet! | |||
Proven Targets:
| CHEMBL ID | UniProt ID | Name | Min activity | Assay type | Source |
|---|---|---|---|---|---|
| No proven targets yet! | |||||
Predicted Targets (via Super-PRED):
| CHEMBL ID | UniProt ID | Name | Probability | Model accuracy |
|---|---|---|---|---|
| CHEMBL5619 | P27695 | DNA-(apurinic or apyrimidinic site) lyase | 98.64% | 91.11% |
| CHEMBL1951 | P21397 | Monoamine oxidase A | 94.81% | 91.49% |
| CHEMBL3108638 | O15164 | Transcription intermediary factor 1-alpha | 94.77% | 95.56% |
| CHEMBL3192 | Q9BY41 | Histone deacetylase 8 | 94.09% | 93.99% |
| CHEMBL3251 | P19838 | Nuclear factor NF-kappa-B p105 subunit | 93.61% | 96.09% |
| CHEMBL2581 | P07339 | Cathepsin D | 93.50% | 98.95% |
| CHEMBL1293249 | Q13887 | Kruppel-like factor 5 | 93.12% | 86.33% |
| CHEMBL5608 | Q16288 | NT-3 growth factor receptor | 92.39% | 95.89% |
| CHEMBL5697 | Q9GZT9 | Egl nine homolog 1 | 91.15% | 93.40% |
| CHEMBL3060 | Q9Y345 | Glycine transporter 2 | 90.74% | 99.17% |
| CHEMBL2007 | P16234 | Platelet-derived growth factor receptor alpha | 88.37% | 91.07% |
| CHEMBL226 | P30542 | Adenosine A1 receptor | 87.38% | 95.93% |
| CHEMBL2335 | P42785 | Lysosomal Pro-X carboxypeptidase | 87.27% | 100.00% |
| CHEMBL1841 | P06241 | Tyrosine-protein kinase FYN | 86.69% | 81.29% |
| CHEMBL3137262 | O60341 | LSD1/CoREST complex | 85.33% | 97.09% |
| CHEMBL241 | Q14432 | Phosphodiesterase 3A | 84.80% | 92.94% |
| CHEMBL2073 | P07947 | Tyrosine-protein kinase YES | 84.46% | 83.14% |
| CHEMBL2373 | P21730 | C5a anaphylatoxin chemotactic receptor | 84.07% | 92.62% |
| CHEMBL1860 | P10827 | Thyroid hormone receptor alpha | 83.94% | 99.15% |
| CHEMBL3401 | O75469 | Pregnane X receptor | 83.85% | 94.73% |
| CHEMBL5469 | Q14289 | Protein tyrosine kinase 2 beta | 83.67% | 91.03% |
| CHEMBL2635 | P51452 | Dual specificity protein phosphatase 3 | 82.40% | 94.00% |
| CHEMBL253 | P34972 | Cannabinoid CB2 receptor | 81.87% | 97.25% |
| CHEMBL2056 | P21728 | Dopamine D1 receptor | 81.78% | 91.00% |
| CHEMBL4478 | Q00975 | Voltage-gated N-type calcium channel alpha-1B subunit | 81.71% | 97.14% |
| CHEMBL233 | P35372 | Mu opioid receptor | 80.71% | 97.93% |
| CHEMBL1902 | P62942 | FK506-binding protein 1A | 80.38% | 97.05% |
| CHEMBL4261 | Q16665 | Hypoxia-inducible factor 1 alpha | 80.15% | 85.14% |
Below are displayed all the plants proven (via scientific papers) to contain this
compound!
To see more specific details click the taxa you are interested in.
To see more specific details click the taxa you are interested in.
| Endosamara racemosa |
| PubChem | 11724813 |
| LOTUS | LTS0161992 |
| wikiData | Q105000625 |