| 115-69-5 |
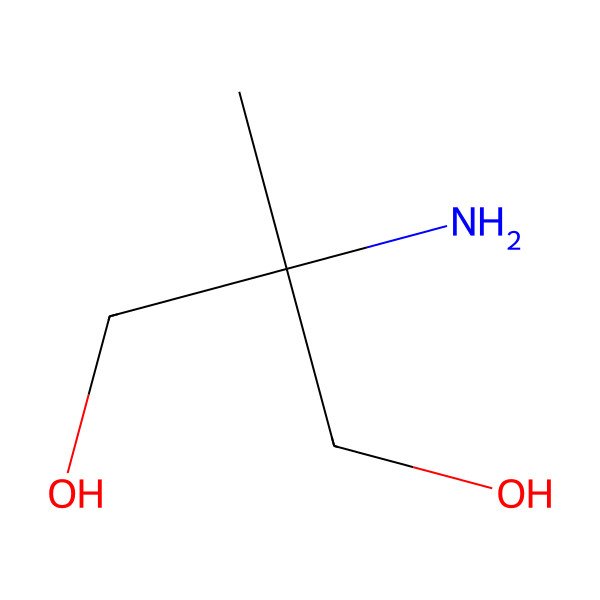
| 2-Amino-2-methylpropane-1,3-diol |
| AMPD |
| Aminoglycol |
| Aminomethyl propanediol |
| 1,3-Propanediol, 2-amino-2-methyl- |
| Isobutandiol-2-amine |
| Ammediol |
| 1,1-Di(hydroxymethyl)ethylamine |
| 2-Amino-2-methyl-1,3-propandiol |
| Amediol |
| 2-AMINO-2-METHYLPROPANEDIOL |
| NSC 6364 |
| AMPO |
| CZ7BU4QZJZ |
| UNII-CZ7BU4QZJZ |
| EINECS 204-100-7 |
| BRN 0635708 |
| AI3-03949 |
| 2-Amino-2-methylpropan-1,3-diol |
| 2-methyl-2-amino-1,3-propanediol |
| NSC-6364 |
| 1,3-Dihydroxy-2-methyl-2-propylamine |
| 1,3-Dihydroxy-2-amino-2-methylpropane |
| 4-04-00-01881 (Beilstein Handbook Reference) |
| MFCD00004678 |
| 2-amino-2-methyl-propane-1,3-diol |
| 1, 2-amino-2-methyl- |
| SCHEMBL23687 |
| CHEBI:991 |
| 11-Di(hydroxymethyl)ethylamine |
| DTXSID7059430 |
| WLN: Q1XZ1 & 1Q |
| 1 1-Di(hydroxymethyl)ethylamine |
| UXFQFBNBSPQBJW-UHFFFAOYSA- |
| 1,1-di (hidroximetil) etilamina |
| NSC6364 |
| 2-amino-2-methyl-1,3propanediol |
| 2-amino-2-methylpropane-1,3diol |
| 2-amino-2-metilpropan-1,3-diol |
| 2-amino-2-methyl-1-3-propandiol |
| 2-Amino-2-methylpropan-1 3-diol |
| 2-metil-2-amino-1,3-propanodiol |
| Amino-2 mthyl-2 propanediol-1,3 |
| 2-amino-2-methylpropane 1,3-diol |
| AMY14265 |
| 2-Amino-2-methyl-1 3-propanediol |
| 2-hydroxymethyl-2-amino-1-propanol |
| 2-Methyl-2-amino-1 3-propanediol |
| BBL027473 |
| STL373468 |
| 1,3-propanodiol, 2-amino-2-metil- |
| 2-amino-2-metil-1, 3--propanodiol |
| AMINOMETHYL PROPANEDIOL [INCI] |
| 1,3-dihidroxi-2-metil-2-propilamina |
| 13-Dihydroxy-2-amino-2-methylpropane |
| AKOS006222995 |
| 1 3-Dihydroxy-2-methyl-2-propylamine |
| 1,3-dihidroxi-2-amino-2-metilpropano |
| AT27658 |
| CS-W017770 |
| HY-W017054 |
| SB83841 |
| 1 3-Dihydroxy-2-amino-2-methylpropane |
| 1,3-dihydroxy-2-methyl-2-amino-propane |
| VS-08545 |
| LS-120137 |
| 2- amino- 2- methylpropane- 1, 3- diol |
| A0332 |
| FT-0611017 |
| 2-Amino-2-methyl-1,3-propanediol, >=99% |
| EN300-93582 |
| PROPANE-1,3-DIOL, 2-AMINO-2-METHYL- |
| 2-AMINO-2-METHYL-1,3-PROPANEDIOL [MI] |
| 2 - amino - 2 - methylpropane - 1,3 - diol |
| J-003318 |
| Q3598002 |
| 2-Amino-2-methyl-1,3-propanediol, >=97.0% (GC) |
| F2190-0374 |
| 95E0210B-CCBA-4485-9DA0-34844FF88E42 |
| 2-Amino-2-methyl-1,3-propanediol, BioUltra, >=99.5% (NT) |
| InChI=1/C4H11NO2/c1-4(5,2-6)3-7/h6-7H,2-3,5H2,1H3 |
| 2-Amino-2-methyl-1,3-propanediol, BioXtra, pH 10.0-12.0 (20 C, 0.5 M in H2O), >=99% |
|
There are more than 10 synonyms. If you wish to see them all click here.
|