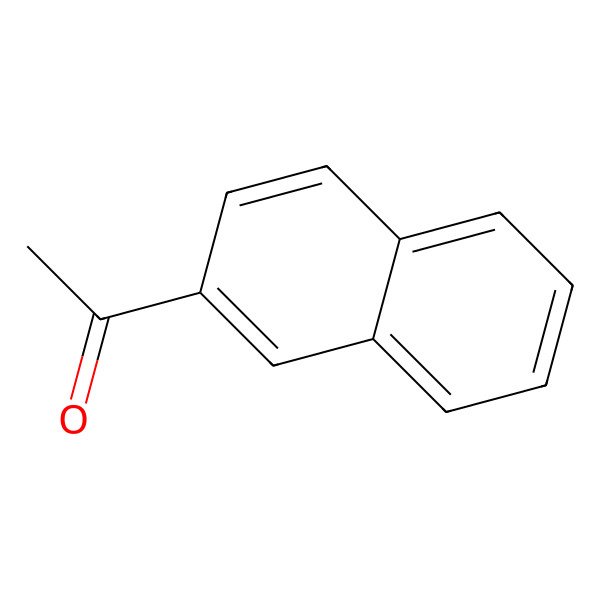
| 2-Acetonaphthone |
| 93-08-3 |
| 2'-Acetonaphthone |
| 1-(naphthalen-2-yl)ethanone |
| Methyl 2-naphthyl ketone |
| Ethanone, 1-(2-naphthalenyl)- |
| Acetonaphthone |
| 1-(2-Naphthyl)ethanone |
| 2-Naphthyl methyl ketone |
| Methyl beta-naphthyl ketone |
| Oranger cyrstals |
| 1-naphthalen-2-ylethanone |
| beta-Acetonaphthalene |
| 1-(2-NAPHTHALENYL)ETHANONE |
| Oranger crystals |
| Ketone, methyl 2-naphthyl |
| Cetone D |
| beta-Acetonaphthone |
| 1-(Naphthyl)ethan-1-one |
| beta-Acetylnaphthalene |
| beta-Naphthyl methyl ketone |
| 1333-52-4 |
| .beta.-Acetonaphthone |
| FEMA No. 2723 |
| 1-(naphthalen-2-yl)ethan-1-one |
| NSC 7658 |
| 2-ACETYL NAPHTHALENE |
| Methyl .beta.-naphthyl ketone |
| CCRIS 6558 |
| .beta.-Acetonaphthalene |
| .beta.-Acetylnaphthalene |
| EINECS 202-216-2 |
| MFCD00004108 |
| 1-naphthalen-2-yl-ethanone |
| BRN 0774965 |
| UNII-21D49LOP2T |
| .beta.-Methyl naphthyl ketone |
| .beta.-Naphthyl methyl ketone |
| AI3-00642 |
| 21D49LOP2T |
| DTXSID2041389 |
| CHEBI:52364 |
| NSC-7658 |
| EC 202-216-2 |
| 4-07-00-01294 (Beilstein Handbook Reference) |
| 2'-acetonaphthon |
| 2-acetylnaphthlene |
| 2-Acetonaphthanone |
| 2-acetyl napthalene |
| beta -acetonaphthone |
| 2-acetyl-naphthalene |
| 2' - acetonaphthone |
| beta -acetonaphthalene |
| beta -acetylnaphthalene |
| 2-Acetonaphthone, 99% |
| Methyl beta-naphthylketone |
| HGY15VJE1U |
| 1-(naphthalenyl)-ethanone |
| 1-(2-Naphthyl)ethanone # |
| beta -methyl naphthyl ketone |
| beta -naphthyl methyl ketone |
| Methyl beta -naphthyl ketone |
| SCHEMBL43058 |
| 1-(2-naphthalenyl)-ethanone |
| 1-(naphthalen-3-yl)ethanone |
| BETA.-ACETYLNAPHTHALENE |
| WLN: L66J CV1 |
| Etanona, 1-(2-naftalenil)- |
| 2-ACETONAPHTHONE [INCI] |
| CHEMBL3183700 |
| DTXCID0021389 |
| NSC7658 |
| AMY38256 |
| CS-B1305 |
| HY-Y1819 |
| BETA.-NAPHTHYL METHYL KETONE |
| Tox21_302186 |
| STK370453 |
| 2-Acetonaphthone, analytical standard |
| AKOS000119679 |
| LS-2943 |
| CAS-93-08-3 |
| s12359 |
| NCGC00257541-01 |
| AC-26572 |
| AS-14446 |
| METHYL BETA-NAPHTHYL KETONE [FCC] |
| PD158455 |
| A0053 |
| FT-0610939 |
| Methyl 2-naphthyl ketone 2-Acetylnaphthalene |
| EN300-20021 |
| METHYL .BETA.-NAPHTHYL KETONE [FHFI] |
| Methyl beta-naphthyl ketone, >=99%, FCC, FG |
| A844438 |
| AC-907/25014303 |
| W-100256 |
| Q27123397 |
| F1995-0259 |
| Z104476442 |
| InChI=1/C12H10O/c1-9(13)11-7-6-10-4-2-3-5-12(10)8-11/h2-8H,1H |
|
There are more than 10 synonyms. If you wish to see them all click here.
|