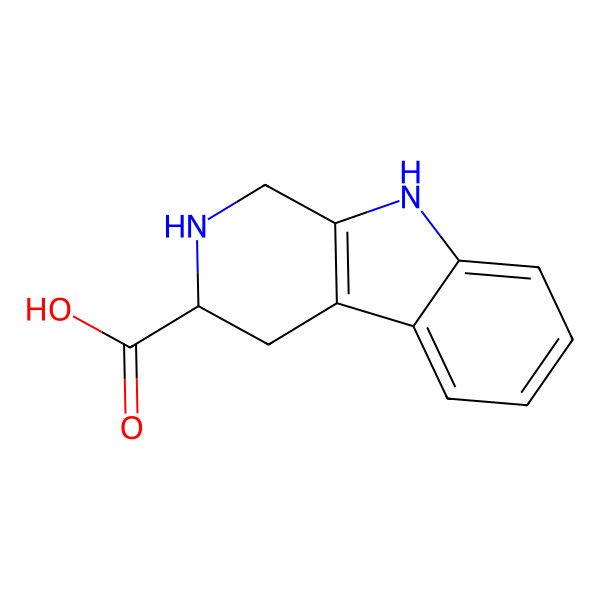
| 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid |
| 2,3,4,9-Tetrahydro-1H-beta-carboline-3-carboxylic acid |
| 1,2,3,4-Tetrahydro-beta-carboline-3-carboxylic acid |
| 1H,2H,3H,4H,9H-pyrido[3,4-b]indole-3-carboxylic acid |
| CCRIS 6486 |
| NSC 96912 |
| CK2W5X3J2K |
| Tetrahydro-beta-carboline-3-carboxylic acid |
| MFCD00204332 |
| NSC-96912 |
| L-1,2,3,4-Tetrahydro-beta-carboline-3-carboxylic acid |
| 2,3,4,9-TETRAHYDRO-1H-b-CARBOLINE-3-CARBOXYLIC ACID |
| H-D-Tpi-OH;D-1,2,3,4-Tetrahydro-beta-carboline-3-carboxylic acid;D-Tryptoline-3-carboxylic acid;D-1,2,3,4-Tetrahydro-9H-pyrido[3,4-b]indole-3-carboxylic acid |
| Maybridge1_002151 |
| H-Tpi-OH;L-1,2,3,4-Tetrahydro-beta-carboline-3-carboxylic acid;L-Tryptoline-3-carboxylic acid;L-1,2,3,4-Tetrahydro-9H-pyrido[3,4-b]indole-3-carboxylic acid |
| 1H-Pyrido(3,4-b)indole-3-carboxylic acid, 2,3,4,9-tetrahydro- |
| DL-Tpi-OH |
| ChemDiv1_000471 |
| UNII-CK2W5X3J2K |
| CBDivE_002277 |
| CBDivE_005090 |
| SCHEMBL416558 |
| CHEMBL149177 |
| beta-Carbocine-3-carboxylic acid |
| CHEBI:91151 |
| FSNCEEGOMTYXKY-UHFFFAOYSA- |
| HMS547J17 |
| HMS588F09 |
| DTXSID401032096 |
| .beta.-Carbocine-3-carboxylic acid |
| NSC96912 |
| CCG-41295 |
| STK387520 |
| AKOS000273957 |
| AKOS016038051 |
| Carboline base + 4H, carboxylic acid |
| AM83757 |
| MS-1161 |
| SB45333 |
| SDCCGMLS-0065883.P001 |
| LS-133456 |
| TRYPTOPHAN IMPURITY H [EP IMPURITY] |
| 3-Carboxy-1,2,3,4-tetrahydro-2-carboline |
| BB 0218166 |
| CS-0020443 |
| EU-0001445 |
| FT-0651518 |
| FT-0651519 |
| FT-0705298 |
| 3-Carboxy-1,2,3,4-tetrahydro-beta-carboline |
| EN300-111082 |
| AF-399/25070003 |
| D-1,2,3,4-tetrahydronorharmane-3-carboxylicacid |
| SR-01000399955 |
| (-)-3-Carboxy-1,2,3,4-tetrahydro-beta-carboline |
| N-ACETYLTRYPTOPHAN IMPURITY H [EP IMPURITY] |
| SR-01000399955-1 |
| SR-01000399955-2 |
| DL-1,2,3,4-TETRAHYDRO-3-CARBOXY-2-CARBOLINE |
| Q27163087 |
| Z56762879 |
| 2,3,4,9-Tetrahydro-1H-ss-carboline-3-carboxylic acid |
| F0037-1026 |
| (+/-)-1,2,3,4-TETRAHYDRO-3-CARBOXY-2-CARBOLINE |
| 1,2,3,4-tetrahydro-pyrido[3,4-b]indole-3-carboxylic acid |
| 2,3,4,9-Tetrahydro-1H-beta-carboline-3-carboxylic acid # |
| (3RS)-1,2,3,4-tetrahydro-beta-carboline-3-carboxylic acid |
| 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylicacid |
| (3RS)-1,2,3,4-TETRAHYDRO-9H-.BETA.-CARBOLINE-3-CARBOXYLIC ACID |
| InChI=1/C12H12N2O2/c15-12(16)10-5-8-7-3-1-2-4-9(7)14-11(8)6-13-10/h1-4,10,13-14H,5-6H2,(H,15,16) |
|
There are more than 10 synonyms. If you wish to see them all click here.
|