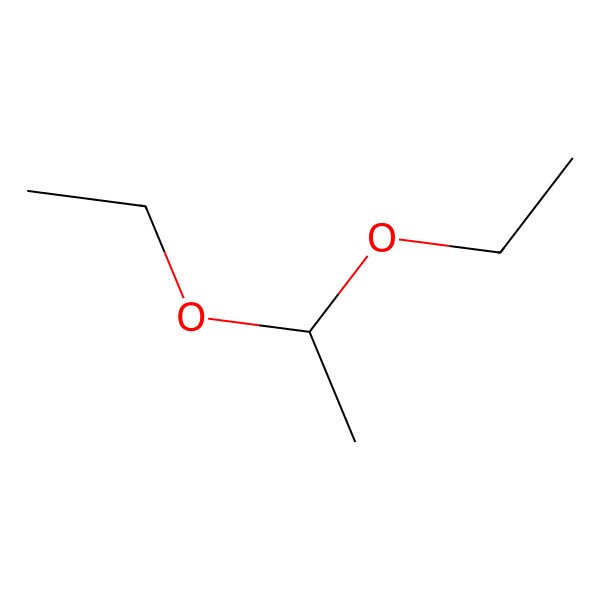
| 1,1-DIETHOXYETHANE |
| 105-57-7 |
| Acetaldehyde diethyl acetal |
| Diethyl acetal |
| Diethylacetal |
| Ethane, 1,1-diethoxy- |
| Diaethylacetal |
| Acetale |
| Ethylidene diethyl ether |
| Acetal diethylique |
| 1,1-Dietossietano |
| Acetaal |
| 1,1-Diethoxy-ethaan |
| 1,1-Diaethoxy-aethan |
| Acetaldehyde, diethyl acetal |
| Ethane, diethoxy- |
| Ethylidenediethyl ether |
| Acetaal [Dutch] |
| Acetaldehyde ethyl acetal |
| Acetal (natural) |
| Acetale [Italian] |
| USAF DO-45 |
| 1,1-diethoxy-ethane |
| FEMA No. 2002 |
| Diaethylacetal [German] |
| Capsicum annuum l |
| NSC 7624 |
| Acetal diethylique [French] |
| 1,1-Dietossietano [Italian] |
| 1,1-Diethoxyacetal |
| 1,1-Diethoxy-ethaan [Dutch] |
| 1,1-Diaethoxy-aethan [German] |
| Diethoxy-1,1-ethane |
| HSDB 1635 |
| Ethylidine diethyl ether |
| acetaldehyde diethylacetal |
| EINECS 203-310-6 |
| UN1088 |
| BRN 1098310 |
| UNII-5G14F9E2HB |
| CH3CH(OC2H5)2 |
| AI3-24135 |
| 5G14F9E2HB |
| DTXSID6030607 |
| NSC-7624 |
| Acetal (Acetaldehyde diethyl acetal) |
| 30846-29-8 |
| Acetal [UN1088] [Flammable liquid] |
| NCGC00091076-01 |
| ACETALDEHYDE-D4 DIETHYL ACETAL |
| DTXCID4010607 |
| C6H14O2 |
| diethoxyethane |
| CAS-105-57-7 |
| 352438-71-2 |
| diethoxy-ethane |
| Acetal Natural |
| Acetal resin |
| Cadco acetal |
| Acetron GP |
| Aceton NS |
| Delrin AF Blend |
| Acetal (VAN) |
| MFCD00009243 |
| Delrin 100ST |
| Delrin 150SA |
| Delrin 500T |
| Delrin 550SA |
| Delrin 100 |
| Delrin 107 |
| Delrin 500 |
| Delrin 507 |
| Delrin 570 |
| Delrin 900 |
| Diaethylacetal(german) |
| Ethanal diethyl acetal |
| Ethylene diethyl ether |
| 1, 1-Diethoxyethane |
| 1,1 - diethoxyethane |
| AEL (CHRIS Code) |
| Thermocomp KB-1008 |
| Etano, 1,1-dietoxi- |
| ACETAL [FHFI] |
| ACETAL [HSDB] |
| ACETAL [MI] |
| Dithylactal de l'actaldhyde |
| SCHEMBL9459 |
| Acetal, >=98%, FG |
| AT-20GF |
| Delrin 100AF, 500AF |
| CHEMBL1338583 |
| FEMA 2002 |
| Acetal, natural, FG, >=97% |
| NSC7624 |
| WLN: 2OY1 & O2 |
| 1, 1-Diaethoxy-aethan(GERMAN) |
| Acetaldehyde diethyl acetal, 99% |
| CHEBI:179533 |
| (C6-H14-O2)x- |
| Electrafil J-80/CF/10/TF/10 |
| Tox21_111078 |
| Tox21_200274 |
| BBL011492 |
| NA1088 |
| STL146604 |
| AKOS005721110 |
| LS-2076 |
| UN 1088 |
| Acetal [UN1088] [Flammable liquid] |
| ACETALDEHYDE DIETHYL ACETAL [FCC] |
| NCGC00091076-02 |
| NCGC00257828-01 |
| VS-02960 |
| N,N-Diethyl-N,N-dibenzyl ammonium chloride |
| D0455 |
| FT-0606113 |
| EN300-20127 |
| Acetaldehyde diethyl acetal, analytical standard |
| Q410938 |
| SR-01000944353 |
| J-001450 |
| J-803005 |
| SR-01000944353-1 |
| Acetaldehyde diethylacetal 100 microg/mL in Acetonitrile |
| F8880-7267 |
| Z104476998 |
| InChI=1/C6H14O2/c1-4-7-6(3)8-5-2/h6H,4-5H2,1-3H |
| 73506-93-1 |
|
There are more than 10 synonyms. If you wish to see them all click here.
|