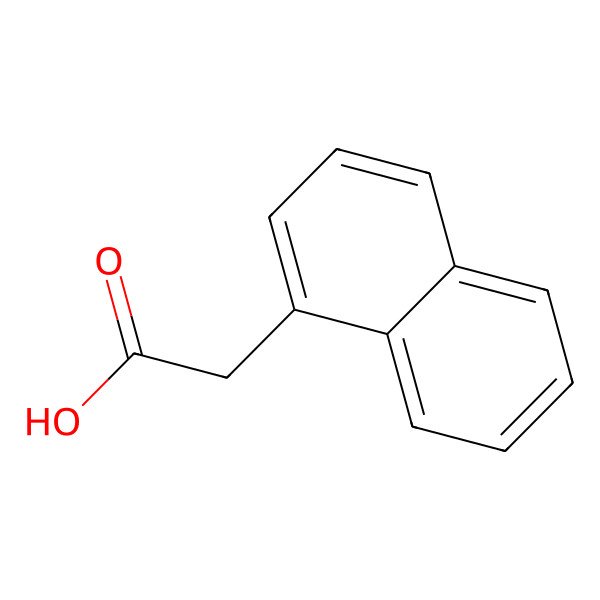
| 86-87-3 |
| 1-NAPHTHALENEACETIC ACID |
| 2-(Naphthalen-1-Yl)Acetic Acid |
| Naphthalene-1-acetic acid |
| Phyomone |
| 1-Naphthalene acetic acid |
| Transplantone |
| NAPHTHALENEACETIC ACID |
| Planofix |
| Fruitone N |
| Fruitofix |
| Planofixe |
| Celmone |
| alpha-Naphthylacetic acid |
| Tre-hold |
| Stop-Drop |
| alpha-Naphthaleneacetic acid |
| alpha-NAA |
| Tip-Off |
| 2-(1-Naphthyl)acetic acid |
| Alphaspra |
| Klingtite |
| Nafusaku |
| Primacol |
| Rhodofix |
| Agronaa |
| Biokor |
| Etifix |
| Pomoxon |
| Stafast |
| Tekkam |
| Vardhak |
| Alman |
| Rasin |
| Liqui-stik |
| Pimacol-Sol |
| Appl-set |
| Rhizopon B |
| Niagara-Stik |
| naphthalen-1-ylacetic acid |
| Nu-Tone |
| Naphthylacetic acid |
| Floramon |
| Hormofix |
| Regenasol |
| Plucker |
| NAA |
| Fruit Fix |
| 1-Naphthtaleneacetic acid |
| NAPHTHALEN-1-YL-ACETIC ACID |
| 1-Naphthylessigsaeure |
| Naphyl-1-essigsaeure |
| STIK |
| Caswell No. 589 |
| 1-NAA |
| .alpha.-NAA |
| 2-naphthalen-1-ylacetic acid |
| .alpha.-Naphthaleneacetic acid |
| 1-naphthyl acetic acid |
| Phymone |
| Germon |
| HSDB 2038 |
| N 10 |
| Stimolante 66f |
| alpha-Naphthyleneacetic acid |
| .alpha.-Naphthylacetic acid |
| NSC 15772 |
| Naphthaleneacetic acid (VAN) |
| Acide naphtylacetique |
| EPA Pesticide Chemical Code 056002 |
| Acide naphtylacetique [French] |
| Naphyl-1-essigsaeure [German] |
| 2-(alpha-Naphthyl)ethanoic aid |
| Acide naphthylacetique |
| Acide naphthylacetique [French] |
| ANU |
| 2-(alpha-Naphthyl)ethanoic acid |
| Kyselina 1-naftyloctova [Czech] |
| Naphthyl-1-essigsaeure [German] |
| AI3-16113 |
| Naphthyl-1-essigsaeure |
| NAA 800 |
| a-naphthaleneacetic acid |
| alpha-Naphthylessigsaeure [German] |
| Kyselina 1-naftyloctova |
| alpha-Naphthylessigsaeure |
| Acide naphtylacetique [ISO-French] |
| EINECS 201-705-8 |
| alpha-naphthyl acetic acid |
| 26445-01-2 |
| .alpha.-Naphthyleneacetic acid |
| CCRIS 8814 |
| CHEMBL428495 |
| DTXSID8020915 |
| UNII-33T7G7757C |
| CHEBI:32918 |
| NSC-15772 |
| 33T7G7757C |
| NCGC00090987-02 |
| NCGC00090987-03 |
| NLA |
| 1-Naphthylacetic acid (JAN) |
| DTXCID60915 |
| (Naphthalen-1-yl)acetic Acid (1-Naphthylacetic Acid) |
| 1-NAPHTHYLACETIC ACID [JAN] |
| CAS-86-87-3 |
| a-naphthylacetic acid |
| 1lrh |
| 1-naphthyleddikesyre |
| naphthyl acetic acid |
| MFCD00004046 |
| NAA (auxin) |
| NAFTAL |
| Fruitone (Salt/Mix) |
| naphthalene acetic acid |
| BLX-NLA |
| (1-naphthyl)acetic acid |
| ?-Naphthaleneacetic Acid |
| 1-naphtalene acetic acid |
| 2-naphthalyl acetic acid |
| Naphtalene-1-acetic acid |
| 1-naphthaleneethanoic acid |
| I+/--Naphthylacetic acid |
| (naphth-1-yl)acetic acid |
| D0L8ZX |
| RAIZON 05 |
| .alpha.-Naphthylessigsaeure |
| 2-(1-naphtyl)acetic acid |
| Oprea1_374703 |
| SCHEMBL35925 |
| WLN: L66J B1VQ |
| MLS001055368 |
| (Naphthalen-1-yl)acetic acid |
| [3H]-NAA |
| 1-Naphthaleneacetic acid, 97% |
| 1-Naphthaleneacetic acid (solid) |
| AMY3378 |
| Anti-allergy drug, NicOx/Biolipox |
| HMS1649K07 |
| HMS3039O06 |
| HMS3604I22 |
| NO-donating therapeutics, Biolipox |
| NAPHTHYLACETIC ACID [MART.] |
| NCX-1510 |
| NSC15772 |
| Tox21_111051 |
| Tox21_400007 |
| AC-640 |
| BBL011795 |
| BDBM50022186 |
| s9362 |
| STL163395 |
| (1-Naphthyl)acetic acid, BSI, ISO |
| 1-NAPHTHALENEACETIC ACID [MI] |
| AKOS000104252 |
| Tox21_111051_1 |
| 1-Naphthalene-4-t-acetic acid (9CI) |
| CCG-266475 |
| CS-6285 |
| DB01750 |
| FS-2548 |
| LS-7536 |
| 1-NAPHTHALENEACETIC ACID [INCI] |
| ALPHA-NAPHTHYLACETIC ACID [HSDB] |
| s12283 |
| NCGC00090987-01 |
| NCGC00090987-04 |
| NCGC00090987-05 |
| NO-donating therapeutics, Biolipox/Nicox |
| 1-Naphthaleneacetic acid, technical grade |
| HY-18570 |
| N 40 |
| PD008449 |
| SMR000677931 |
| 1-Naphthaleneacetic acid (7CI,8CI,9CI) |
| 1-NAPHTHALENEACETIC ACID [WHO-DD] |
| 2-(.ALPHA.-NAPHTHYL)ETHANOIC ACID |
| 2-(1-naphthyl)acetate;1-Naphthylacetic acid |
| FT-0608129 |
| N0005 |
| EN300-18112 |
| D01558 |
| N-1420 |
| NO-donating allergy therapeutics, Biolipox/Nicox |
| A841863 |
| AE-508/13205220 |
| Q161660 |
| J-610083 |
| NO-donating respiratory therapeutics, NicOx/Biolipox |
| Z57169437 |
| F0020-1890 |
| 1-Naphthaleneacetic acid, PESTANAL(R), analytical standard |
| NAPHAZOLINE HYDROCHLORIDE IMPURITY B [EP IMPURITY] |
| OX-NLA (nasal/liposomal formulation, allergic rhinitis/rhinitis) |
| 1-Naphthylacetic acid, 1 mg/mL, BioReagent, plant cell culture tested |
| OX-NLA (nasal/liposomal formulation, allergic rhinitis/rhinitis), Meda |
| 1-Naphthaleneacetic acid, plant cell culture tested, BioReagent, >=95%, crystalline |
| 3-(3-tert-Butoxycarbonylamino-piperidin-1-yl)-2-methyl-propionicacidmethylester |
| Liposomal nitric oxide-donating cetirizine derivative (nasal, rhinitis), Biolipox |
| Nitric oxide-donating cetirizine derivative (liposomal, intranasal, rhinitis), Orexo |
|
There are more than 10 synonyms. If you wish to see them all click here.
|